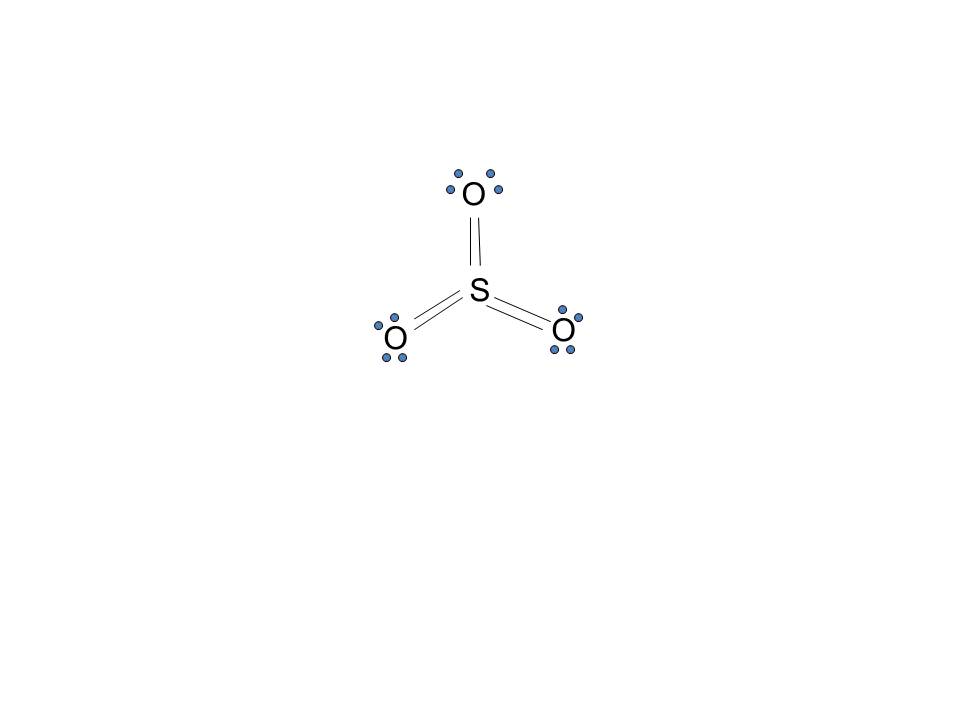
43 lewis dot diagram for so3
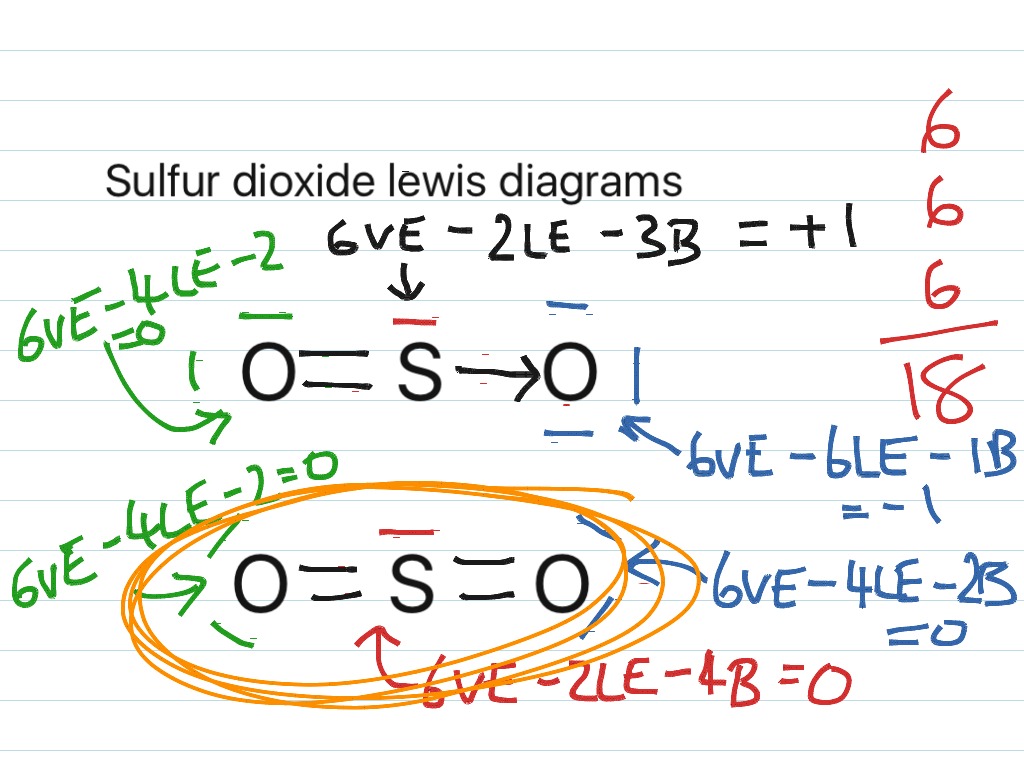
Let's do the SO3 2- Lewis structure. For the SO3 2- compound, we have 26 total valence electrons, and that includes these two electrons up here--there are two extra valence electrons. So we have 26. Let's put the Sulfur at the center and the Oxygens around the outside. Put two electrons between the atoms to form chemical bonds. We've used 6. Dec 02, 2021 · Lewis structure of SO3 The sulfur trioxide is a tetra atomic chemical molecule where both the sulfur and three oxygen molecules bond with an equal number of valence electrons. The diagram is drawn showing dots of valence electrons around the symbol of both sulfur and oxygen atoms with lines predicting bond formation.
SO3 Molecular Geometry, Lewis Structure, and Polarity Explained. SO3 stands for Sulfur Trioxide. This is one of the most pollutant chemical compounds in the gaseous form. It is also a primary agent in the acid rain. The main use of this component is to make sulfuric acid for industrial purposes.

Lewis dot diagram for so3
BeF2 molecule e- tally Lewis dot diagram VSEPR shape Drawn, and named, bond angle stated and . A bond between 2 nonmetal atoms that have the same electronegativity and therefore have equal sharing of the bonding electron pair. See below. For each of the following molecules, draw the Lewis Diagram and tally up the electron pairs. In the SO3 Lewis structure diagram, the sulfur atom can be the center atom of the molecule. As a result, central sulfur in the SO3 Lewis structure, with all three oxygen atoms arranged in trigonal planar geometry. Add valence electrons around the oxygen atom, as given in the figure. Step-3: Lewis dot Structure for SO3 generated from step-1 and ... A step-by-step explanation of how to draw the SO3 Lewis Dot Structure (Sulfur trioxide).For the SO3 structure use the periodic table to find the total number...
Lewis dot diagram for so3. How to draw the Lewis Structure of SO3 (sulfur trioxide) - with explanationSulfur is an exception to the octet rule - it can handle up to 12 electrons!Check ... Lewis structure of SO3 The sulfur trioxide is a tetra atom chemical molecule whereby both the sulfur and also three oxygen molecules bond with an equal variety of valence electrons. The chart is attracted showing dots the valence electrons roughly the prize of both sulfur and also oxygen atoms through lines predicting link formation. A Lewis dot structure is also called a Lewis structure, a Lewis dot diagram, an electron dot structure, or Oct 18, 2021 · Worksheets are Lewis dot structures and molecule geometries work 5 11a electron diagrams and lewis structures wkst key Practice problems h s so ch br hcn Lewis structures shapes and polarity Work 13 Ws lewis structures covalent Lewis. The Lewis structures of Nitrogen Monoxide NO are drawn. 1.S. 2.Total valence electron is 6+3x6 - (-2)= 26. 3.Connect the atoms with single bonds. 4.P=6n+2 -V =6x4 +2 -26 = 26-26 = 0 ; 0 electron which must be shared through pi bonding and unshared i.e; Therefore, there is 0 double bond and an electron.. 5.Distribution of other electron.
Sulfur Trioxide (SO 3) Lewis Structure, Hybridization | Drawing Steps. Sulfur trioxide molecule contains one sulfur atom and three oxygen atoms. We will construct the lewis structure of SO 3 molecule by following VSEPR theory rules and considering stability of intermediate structures. Finally, after obtaining the lewis structure of SO 3, we can determine the hybridization of atoms. Automatic procedure to construct Lewis dot structures, so32-, pi an d - Sulfite Ion SO3-2 Lewis structure, draw the lewis structure for the sulfite ion, Dot structure SO3-, so3 2-, SO32-,Sulfite,Ion,Lewis,dot,structure,hybridization,chemistry,bond,angle,drawing, ib, ap, a, SO3 2- Lewis Structure,Lewis Structure for SO3 2-,Lewis Structure,SO3 2-,SO3 2- Electron Dot Structure,Electron Dot ... Lewis Diagram For Seo3. A Lewis dot structure for SeO3 is drawn with an Se in the center, with two lines connecting it to two Os and one double line connecting it to an O. The Os. Draw all possible resonance structures for the molecule selenium trioxide (SeO3) . The structure and arrows are given, simply add the remaining bonds and lone. (1 ... Write Lewis structures for: (please note, none of the solutions are using the expanded octet rule or formal charges) a. \(\ce{PO4^3-}\) c. \(\ce{SO3^2-}\) d. HONO. Answer a. Answer b. Answer c. Click here to see a video of the solution
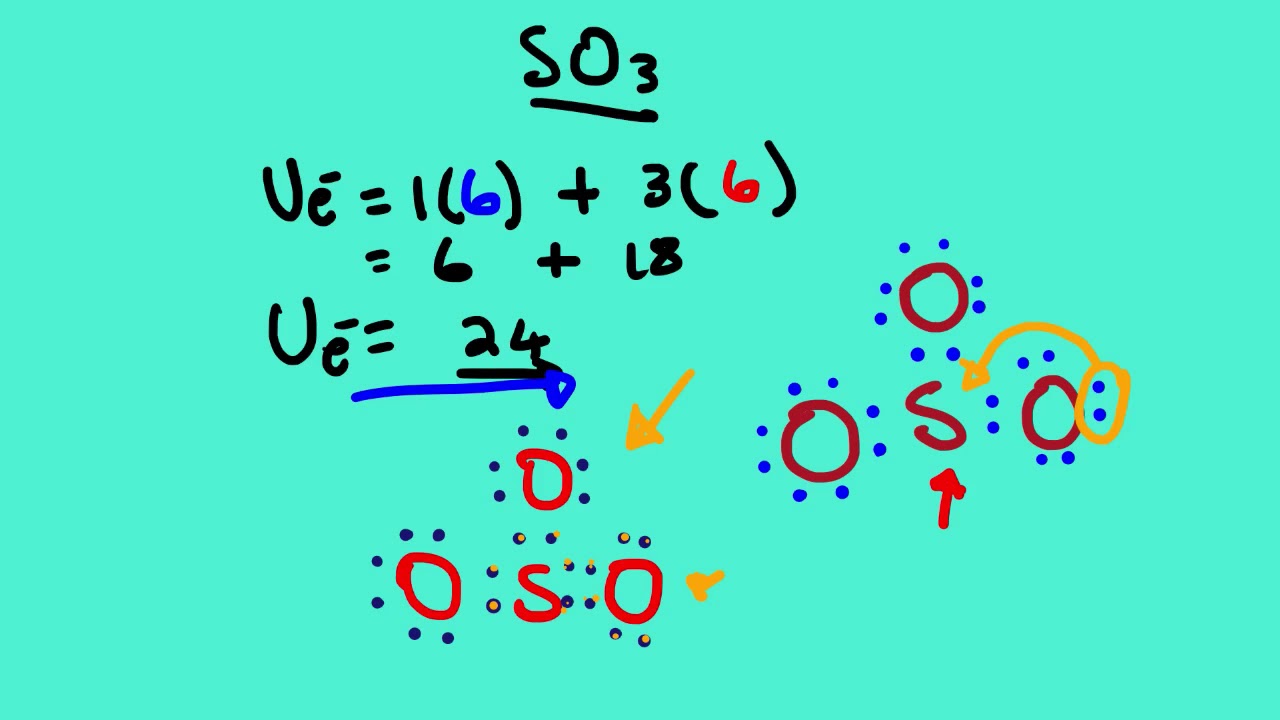
Drawing the Lewis Structure for SO3 (Sulfur Trioxide) It is a form of pollution. SO3 is named Sulfur Trioxide. There are 32 valence electrons available for the Lewis structure for SO3. Be sure to check the formal charges for the Lewis structure for SO3. Click to see complete answer. So 6 minus zero minus 12 over 2; so 6 minus 6 equals zero. So we can write the formal charge for Sulfur as zero. So we have formal charges of zero for each of the atoms in SO3. That makes this the best Lewis structure for SO3. This is Dr. B., and thanks for watching. 27.11.2021 · The Lewis dot structure shows the unpaired electrons or the lone pairs in the end. ... An MO diagram is a descriptive instrument that is particularly used to explain the formation of chemical bonds in molecules with the help of ... Previous Article SO3 Lewis Structure, Molecular Geometry, and Hybridization. Next Article BF3 Lewis ... 2. Draw the Lewis dot structures for each of the following molecules: a. H 2 S c. SO 3 b. CH 2 Br 2 d. HCN 3. Draw the Lewis dot structure for each of the following polyatomic ions: a. NH 4 + c. PO 4 -3 b. NO 3 - d. CO 3 2- 4. For the following molecules or ions (where the central atom is underlined): i. Draw the Electron dot structure. ii.
simple procedure for drawing the SO3 lewis structure, so3 lewis electron dot structure, electrostatic potential, ESP, resonance structures of so3, Lewis structures of SO3| Lewis configuration of sulfur trioxide SO3, octet rule, SO3 Lewis structure, π bonds, 2π electrons, multiple bonds, stable resonance, resonance structures, so3 resonance or double bonds, lewis structures and the octet ...
Drawing the Lewis Structure for SO 3 ( Sulfur Trioxide) SO 3 is the primary contributer to acid rain in the atomsphere. It is a form of pollution. SO 3 is named Sulfur Trioxide. There are 32 valence electrons available for the Lewis structure for SO 3. Be sure to check the formal charges for the Lewis structure for SO 3 .
Phosphite ion lewis structure
Lewis. Dot . Structure (Phosphate ion).For the PO4 3- structure. use the periodic table to find the total num.. Jigzone daily jigsaw puzzle of the day. For the PF4- Lewis structure use the periodic table to find the. In the Lewis structure of PF4-there are a total of 34 valence electrons. Worked example: Lewis diagram of xenon difluoride (XeF₂).
The Lewis Structure, or Lewis Dot Diagram, shows the bonding between atoms of a molecule and any electrons that may exist. The Lewis Structure for Li is Li with one dot to the right of the element.
What is the Lewis dot structure of SO3? It is six for a single sulfur trioxide (SO3) molecule, where both sulfur and each oxygen atom need two valence electrons to stabilize their atom. Next, look for the number and type of bonds forming within a single sulfur trioxide (SO3) molecule.
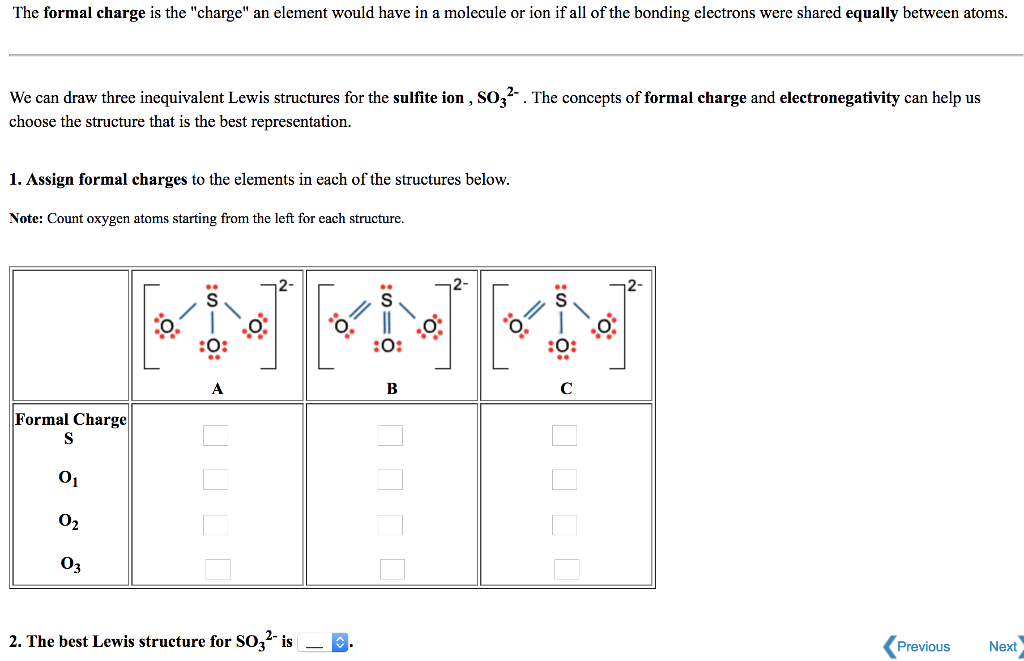
The Lewis Dot Structure for SO 3 2-:. The sulfite anion (SO 3 2-) is present in wines, and is used as preservative in certain foods.The accepted Lewis structure predicts both single and double ...
1.12.2021 · Draw the Lewis dot structures for each of the following molecules: a. Jun 14, 2014 · "CCl"_4 has a tetrahedral geometry with bond angles of 109. Show more To generate the Lewis dot structure, you have to follow the given steps: Find the total count of valence electrons to molecules. So we have 7+7+7+1 = 22 valence electrons. Share.
How many bonds should be drawn in the Lewis-dot structure for SO3? apex users its defenatley 3. Is SO3 a Lewis base or Lewis acid? I think it is acid, because there is a question that asks the ...
Apr 08, 2020 · So3 Lewis Diagrams Wiring Diagrams Folder The lewis dot structure for so3 2. Lewis dot diagram for so3. Lets do the so3 2 lewis structure. So we can write the formal charge for sulfur as zero. For the so3 2 compound we have 26 total valence electrons and that includes these two electrons up here there are two extra valence electrons.
Answer: Please read these instructions, if they do not make sense please PM me and we can set up an online session so I can facetime with you and get you squared away. Alright, here we go! Let's start off by differentiating between SO3 and SO3 2- SO3 is Sulfur Trioxide SO3²¯ is Sulfite They b...
Lewis structures or electron dot structures - SO3 Lewis structure 1. Simple Procedure for writing Lewis Structures - Example: SO3 FOR CLICKABLE LINKS AND A DETAILED DESCRIPTION OF THE SIMPLE METHOD DESCRIBED BELOW PLEASE SEE PAGE 4 OF THIS PRESENTATION A simple procedure for writing Lewis structures is given in a previous article entitled "Lewis Structures and the Octet Rule".
Icl3 lewis structure
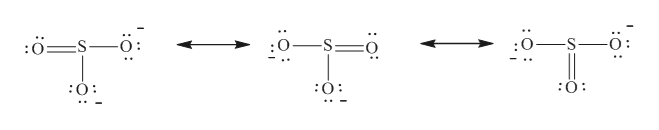
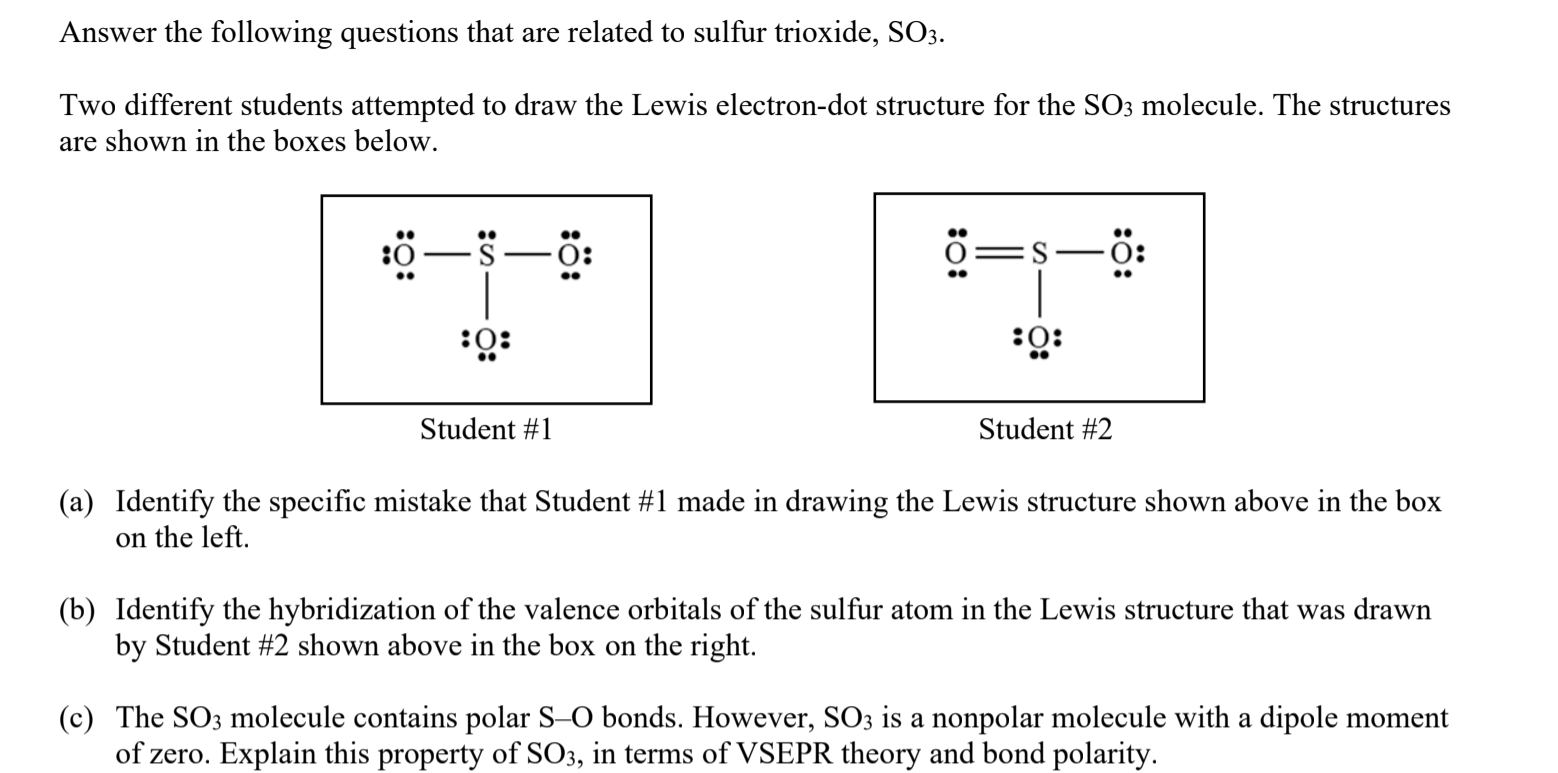
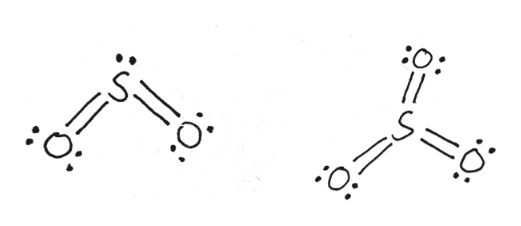
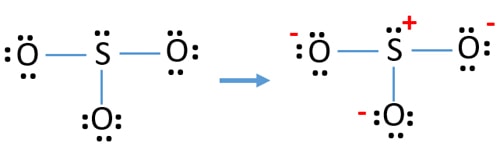
There are seven resonance structures for "SO"_3. > When you draw the Lewis structure, you first get the three structures at the top. In each of them, "S" has a formal charge of +2 and two of the "O" atoms have formal charges of -1. In each of the three structures in the middle, "S" has a formal charge of +1 and one of the "O" atoms has a formal charge of -1.
Are there pi bonds in sulfite Lewis structure? There is only one pi bond and it is located at between one pxygen atom and sulfur atom. Is lewis structure for so32- is different from lewis structure for so42-Yes. They are different. In SO 4 2-lewis structure, there are four oxygen atoms around the sulfur atom. Therefore number of valence ...
Answer (1 of 6): All of the other answers show a structure for SO_3 that has a double bond to each of the three oxygens. This answer for SO_3 comes from a robotic use of formal charge without regard to actual bonding that must be occurring in the molecule. The real problem is this, you cannot sim...
How to draw the Lewis Structure of SO3 sulfur trioxide - with explanationSulfur is an exception to the octet rule - it can handle up to 12 electronsCheck. So3-2 lewis structure. The Lewis Dot Structure for SO3 2-. Chemistry learning made easyThis tutorial will help you deal with the lewis structure and moleculargeometry of sulfite ion SO3 2-.
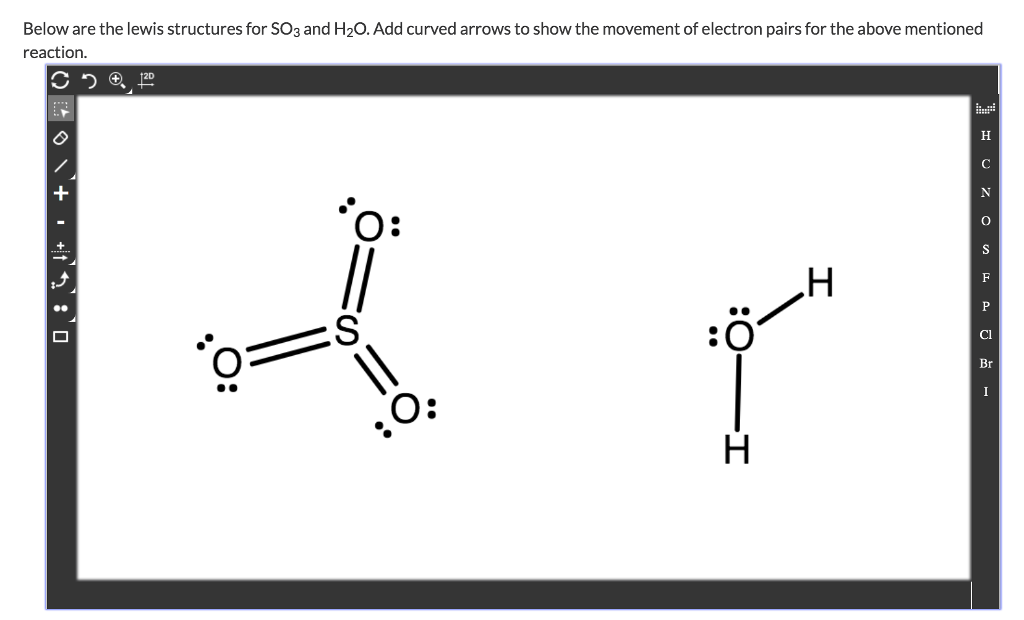
Draw the Lewis structure for the sulfur trioxide (SO{eq}_3{/eq}) molecule. Be sure to include all resonance structures that satisfy the octet rule.
N2 lewis structure
This problem has been solved! Write a single Lewis structure for SO3. Draw the Lewis dot structure for SO3. Include all lone pairs of electrons. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high.
A step-by-step explanation of how to draw the SO3 Lewis Dot Structure (Sulfur trioxide).For the SO3 structure use the periodic table to find the total number...
In the SO3 Lewis structure diagram, the sulfur atom can be the center atom of the molecule. As a result, central sulfur in the SO3 Lewis structure, with all three oxygen atoms arranged in trigonal planar geometry. Add valence electrons around the oxygen atom, as given in the figure. Step-3: Lewis dot Structure for SO3 generated from step-1 and ...
BeF2 molecule e- tally Lewis dot diagram VSEPR shape Drawn, and named, bond angle stated and . A bond between 2 nonmetal atoms that have the same electronegativity and therefore have equal sharing of the bonding electron pair. See below. For each of the following molecules, draw the Lewis Diagram and tally up the electron pairs.







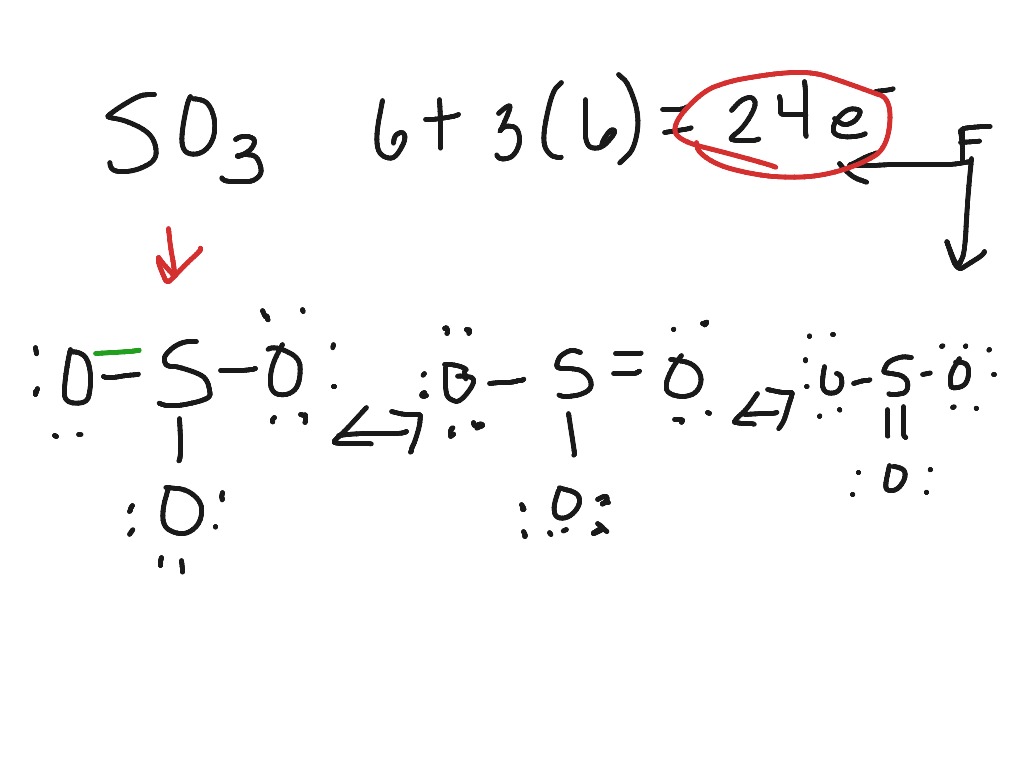








![[CX_4926] Lewis Diagram So32 Schematic Wiring](https://static-cdn.imageservice.cloud/3624270/lewis-diagram-so32-wiring-diagram.jpg)





Comments
Post a Comment