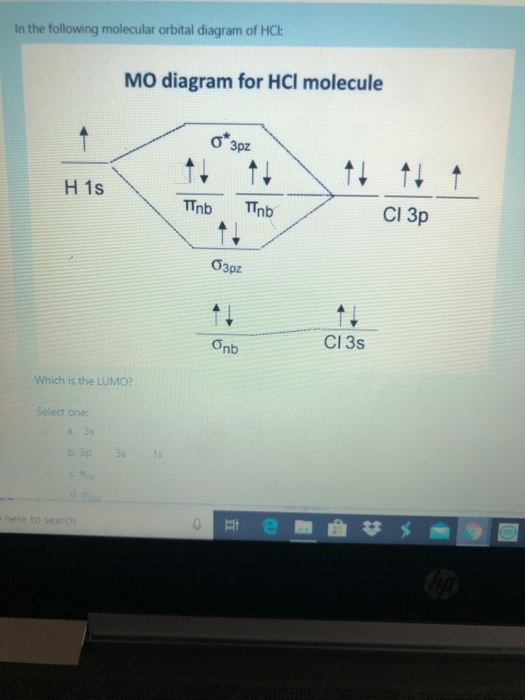
39 mo diagram for hcl
January 31, 2017 - Answer (1 of 2): Here is a useful MO diagram of HCL found on the internet: The Cl electrons residing up to 3s orbital (1s, 2s, 2px,2py,2pz,3s) are largely stabilized than H electron in 1s orbital and therefore they cannot mix and form bond. The 3p electrons of Cl have comparable energy with the ... FREE Answer to Draw an MO diagram for HF and for HCl on the same drawing. Try to make...
An advanced molecular orbital diagram of HF for the inorganic or physical chemistry student.

Mo diagram for hcl
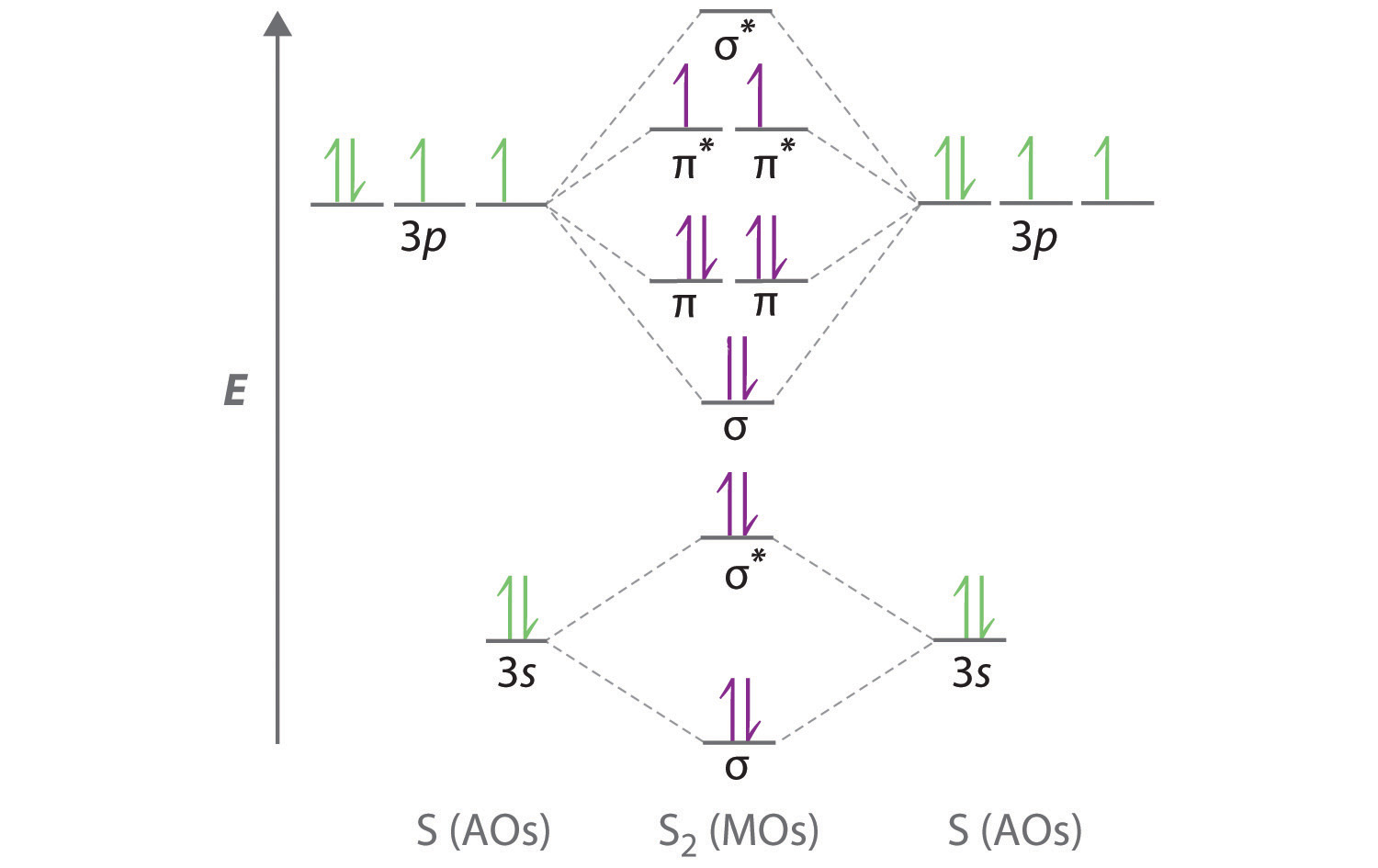
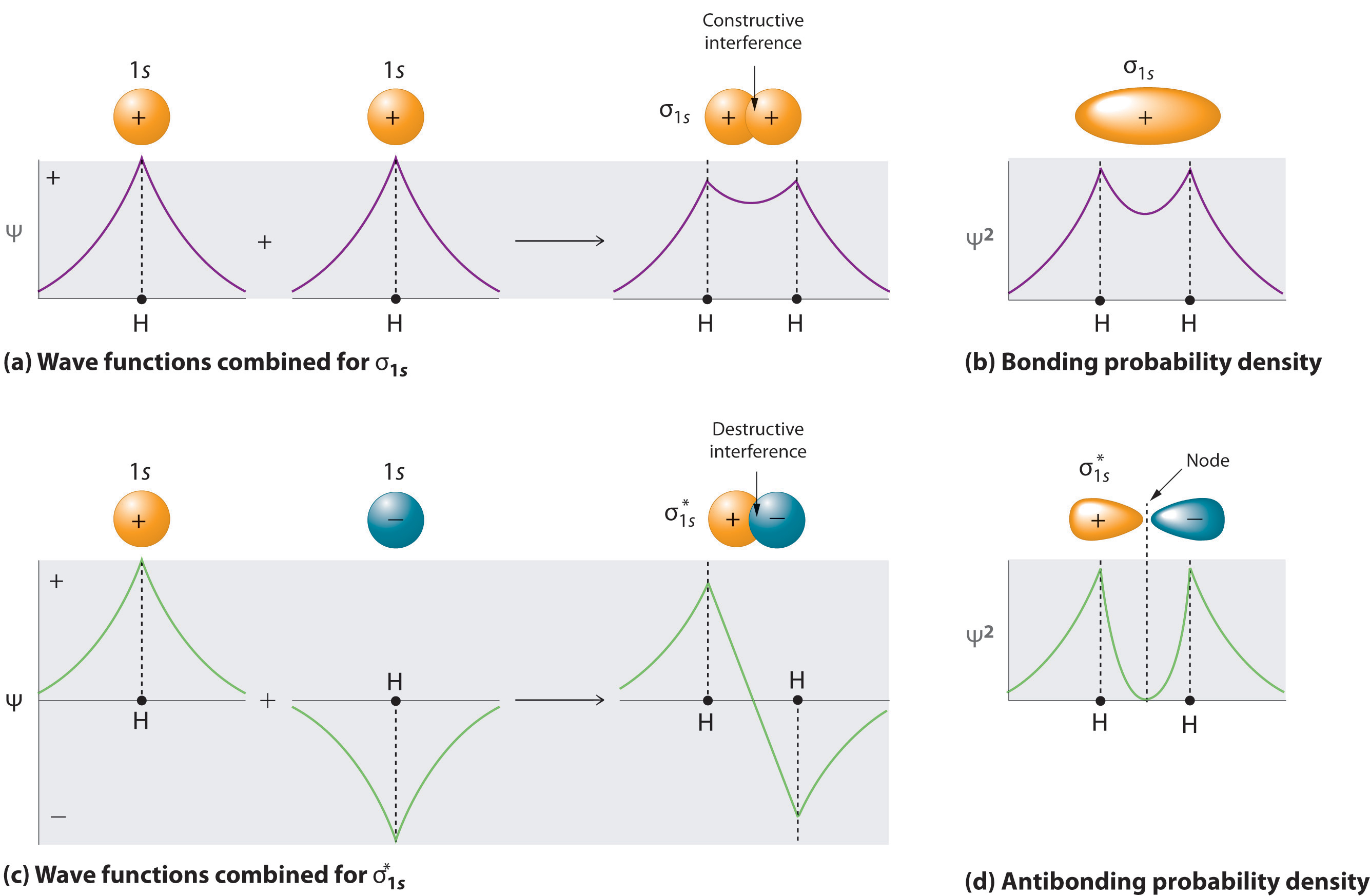
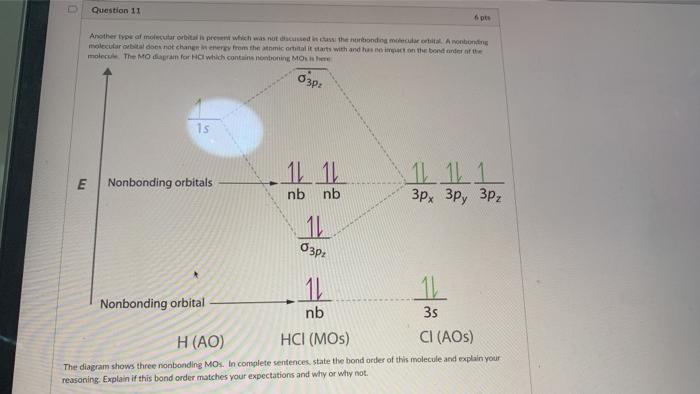
Sulfur (in British English: sulphur) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic.Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S 8.Elemental sulfur is a bright yellow, crystalline solid at room temperature. Sulfur is the tenth most abundant element by mass in the universe and the fifth ... August 11, 2020 - Figure \(\PageIndex{6}\): Molecular Orbital Energy-Level Diagram for HCl. The hydrogen 1s atomic orbital interacts most strongly with the 3pz orbital on chlorine, producing a bonding/antibonding pair of molecular orbitals. The other electrons on Cl are best viewed as nonbonding. Hydrogen chloride has many uses, including cleaning, pickling, electroplating metals, tanning leather, and refining and producing a wide variety of products. Hydrogen chloride can be formed during the burning of many plastics. Upon contact with water, it forms hydrochloric acid. Both hydrogen chloride and hydrochloric acid are corrosive.
Mo diagram for hcl. Inorganic chemistry for upper forms Oscroft, P. W Free. Science education is the process of sharing scientific information with the goal of learning. Chem 410 inorganic chemistry To promote an HCl molecule from the v = 0 to the v = 1 state, we would expect to see an infrared absorption about νo = νe + 2xeνe = 2880 cm−1. However, this absorption corresponding to the Q-branch is not observed due to it being forbidden by symmetry. Instead, two sets of signals (P- ... HCl(aq) + NaOH(aq) --> NaCl(aq) + H 2 O(l) + Energy. Thermochemistry determine the heat exchanged at constant pressure, q = m c ∆T.. Calculating the limiting reactant, the change in enthalpy of the reaction, ∆H rxn, can be determined since the reaction was conducted under conditions of constant pressure ∆H rxn = q rxn / # moles of limiting reactant. This demonstration illustrates how the ... Enjoy the videos and music you love, upload original content, and share it all with friends, family, and the world on YouTube.
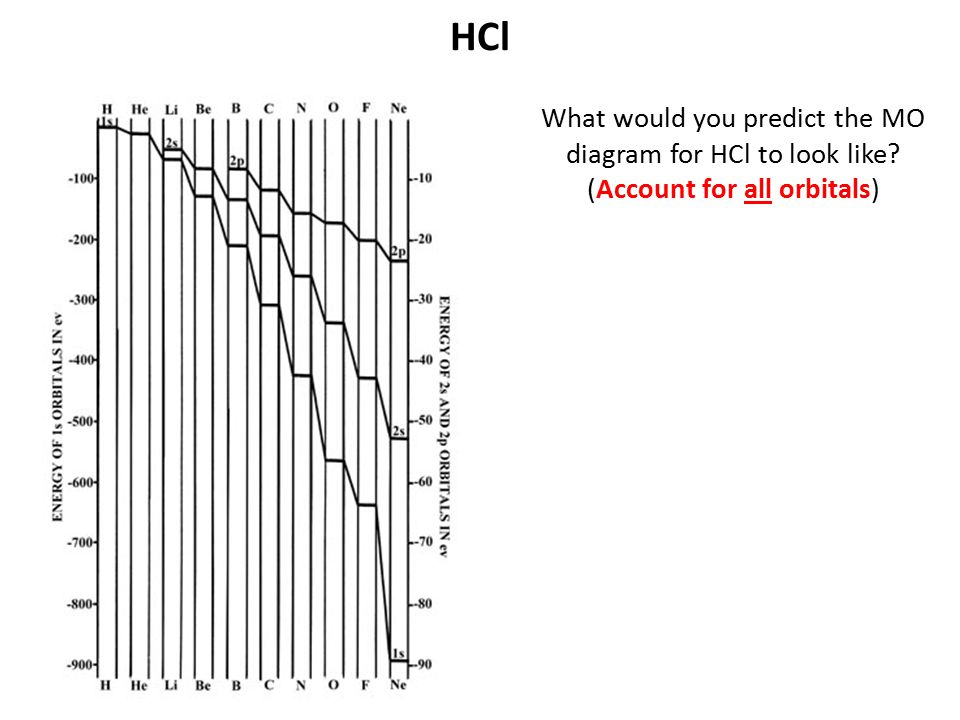
Module1Introduction1.0LearningObjectives随堂测验1、InthefirstvideoofModule1,theexamplesusedtodemonstratet. Module 1 Introduction 1.0 Learning Objectives随堂测验 1、In the first video of Module 1, the examples used to demonstrate the successful application of materials include _____ Draw an MO energy diagram for HCl. Predict the bond order and make a sketch of the lowest energy bonding molecular orbital. Start your trial now! First week only $4.99! arrow_forward. 06/03/2020 · For notes click here-https://trickychemistrysuman.blogspot.com/2020/03/mo-diagram-of-hcl.html Relative AO Energies for MO Diagrams H He Li Be B C N O F Ne B C N O F Ne Na Mg Al Si P S Cl Ar Al Si P S Cl Ar 1s 2s 2p 3s 3p –19.4 eV –15.8 eV –32.4 eV –10.7 eV Photoelectron spectroscopy gives us a pretty good idea of the relative energies for AOs.
Molecular orbital theory is also able to explain the presence of Figure \(\ PageIndex{6}\): Molecular Orbital Energy-Level Diagram for HCl. to describe the bonding in the cyanide ion (CN −). mix atomic orbitals on different atoms to get Molecular Orbitals. The resul7ng MO diagram looks like this. CN– (Cyanide ion), NO+ (Nitrosonium ion). We think about the highest occupied molecular orbital on one reactant as the source of electrons. We imagine the lowest unoccupied orbital on the other reactant as the destination for the electrons. Suppose a proton is transferred from a hydrogen chloride molecule, HCl, to a water molecule, H2O. Complete an MO energy diagram for HCI. Part A Complete an MO energy diagram for HCl. Drag the appropriate labels to their respective targets. Not all labels and targets will be used. Part B View Available Hint(s) Predict the bond order. Express your answer as an integer. View Available Hint(s) 14 ΟΙ ΑΣΦ ? p 1 1 s HCl bond order = 11 11 o ... Examples include CO, HCl, and NO. ... The smallest molecule, hydrogen gas exists as dihydrogen (H-H) with a single covalent bond between two hydrogen atoms. As each hydrogen atom has a single 1s atomic orbital for its electron, the bond forms by overlap of these two atomic orbitals.
How are the 3p orbitals of chlorine lower in energy than the 1s orbital of hydrogen? MO diagram of HCl. Share. Share a link to this question. Copy link
08/07/2020 · This lecture clearly explains the molecular orbital diagram of heteronuclear diatomic molecule (HF & HCl). This will help students of H.S., BS-MS, B. Sc., M....
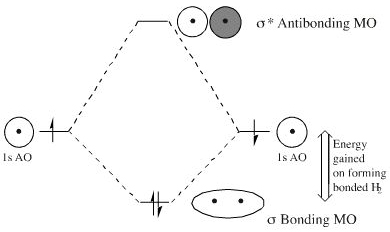
The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 8). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one ...
Physical properties of hydrochloric acid, such as boiling and melting points, density, and pH, depend on the concentration or molarity of HCl in the aqueous solution. They range from those of water at very low concentrations approaching 0% HCl to values for fuming hydrochloric acid at over 40% HCl.
Downloads Hcl Mo Diagram mortgage ... mo diagram for hcl ... Wiring Diagram Of Fender Stratocaster Guitar Wiring Diagrams Single Coil 2005 Club Car Precedent Wiring Diagram Wiring Diagram Honda Vario Diagram Of Freightliner Cascadia Fuse Box Ac 3 Phase Ac Motor Wiring Diagram ...
Symmetry also allows for overlap between the H 1s and F 2pz orbitals, and these two atomic orbitals have a small energy separation; they therefore interact, creating σ and σ* MOs and a molecule with a bond order of one. Hydrogen fluoride: The hydrogen fluoride molecule. Hydrogen chloride, HCl, is ...
Q. Draw the MO energy diagram for HCl on your own, then use it to predict the bond order for the molecule. Q. Draw the MO energy diagram for CO on your own, then use it to predict the bond order for the molecule. (Use the energy ordering of O2. ) Q. What is meant exactly by greater interaction, and why is the answer for this C? ...
25/10/2021 · Problem: Draw the MO energy diagram for HCl on your own, then use it to predict the bond order for the molecule. FREE Expert Solution Show answer Answer: 81% (79 ratings) Sign up for free to keep watching this solution Sign up for free. 579,886. students enrolled. 97%. improved grades . 2,784. minutes of videos. Continue watching with Facebook Continue …
Molecular Orbital Diagrams of Heteronuclear Diatomics. Concept #1: Molecular Orbital Diagrams of Heteronuclear Diatomics. Expert Q&A. Ask unlimited questions and get expert help right away. ... HCl is called hydrogen chloride as a molecule and called hydrochloric acid as an acidd. H2 is a homonuclear diatomic molecule but is not a compounde.
This video explain filling of Molecular orbital in hetronuclear atoms. Energy level Diagram of CO,HF and HCl moleculesEnergy level difference of homoatomic a...
June 2, 2021 - Figure \(\PageIndex{7}\): Molecular orbital energy diagram for the HCl molecule
Chlorine is sp3-hybridized, and so there are four tetrahedrally-arranged 3sp3 orbitals surrounding it.Hydrogen does not hybridize, so it's simply a circle fo...
March 1, 2021 - Figure \(\PageIndex{14}\): Molecular Orbital Energy-Level Diagram for HCl. The hydrogen 1s atomic orbital interacts most strongly with the 3pz orbital on chlorine, producing a bonding/antibonding pair of molecular orbitals. The other electrons on Cl are best viewed as nonbonding.
Hydrogen chloride has many uses, including cleaning, pickling, electroplating metals, tanning leather, and refining and producing a wide variety of products. Hydrogen chloride can be formed during the burning of many plastics. Upon contact with water, it forms hydrochloric acid. Both hydrogen chloride and hydrochloric acid are corrosive.
August 11, 2020 - Figure \(\PageIndex{6}\): Molecular Orbital Energy-Level Diagram for HCl. The hydrogen 1s atomic orbital interacts most strongly with the 3pz orbital on chlorine, producing a bonding/antibonding pair of molecular orbitals. The other electrons on Cl are best viewed as nonbonding.
Sulfur (in British English: sulphur) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic.Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S 8.Elemental sulfur is a bright yellow, crystalline solid at room temperature. Sulfur is the tenth most abundant element by mass in the universe and the fifth ...

















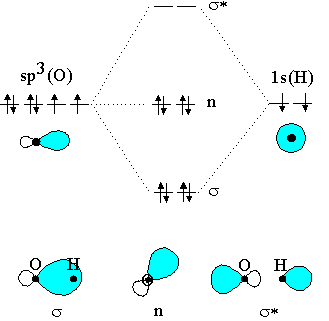



Comments
Post a Comment