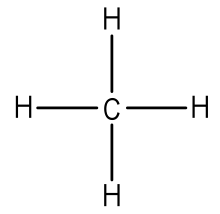
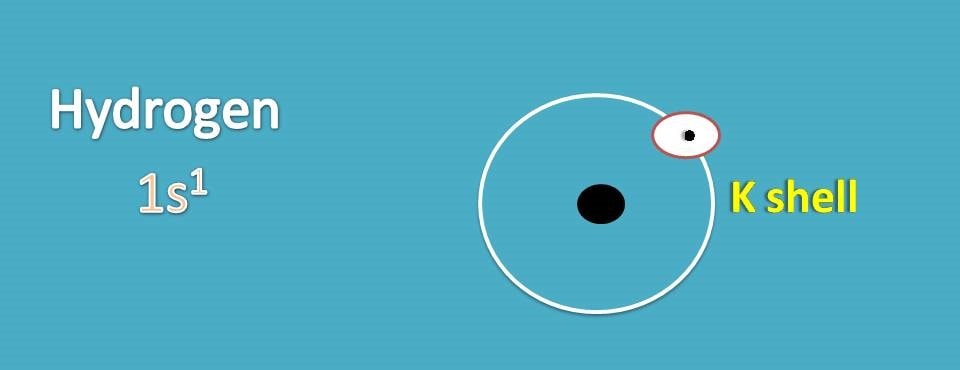
43 lewis dot diagram of hydrogen
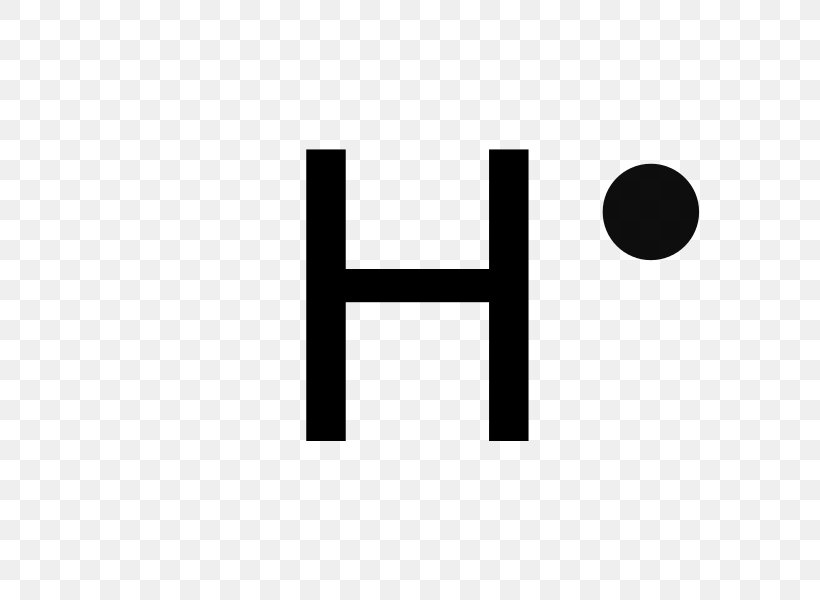
Draw Lewis dot diagram for the following. Hydrogen (H2) Maharashtra State Board HSC Science (General) 11th. Textbook Solutions 8028. Important Solutions 18. Question Bank Solutions 5553. Concept Notes & Videos 418. Syllabus. Advertisement Remove all ads. Draw Lewis dot diagram for the following. ... A step-by-step explanation of how to draw the H+ Lewis Dot Structure.For the H+ structure use the periodic table to find the total number of valence electron...
A step-by-step explanation of how to draw the Lewis dot structure for F (Hydrogen). I show you where Hydrogen is on the periodic table and how to determine ...
Lewis dot diagram of hydrogen
Carbon Monoxide Co Lewis Structure Chemistry Worksheets Lewis Carbon Monoxide . Glow In The Dark Lewis Dot Diagrams Senior Chemistry Quimica Organica Ligacao Quimica Ensino De Quimica . Lithium Electron Configuration Electron Configuration Electrons Diagram Design . Lewis Dot Structure Covalent Bonding Chemistry Worksheets Electron Configuration For atoms with partially filled d or f subshells, these electrons are typically omitted from Lewis electron dot diagrams. For example, the electron dot diagram for iron (valence shell configuration 4s 2 3d 6) is as follows:. Elements in the same column of the periodic table have similar Lewis electron dot diagrams because they have the same valence shell electron configuration. Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place them around the elements symbol. Element Atomic # Atomic Mass Protons Neutrons Electrons Lewis Dot Carbon 6 12 6 6 6 l Hydrogen 1 1 1 0 1 H Lithium 3 7 3 4 3 Li
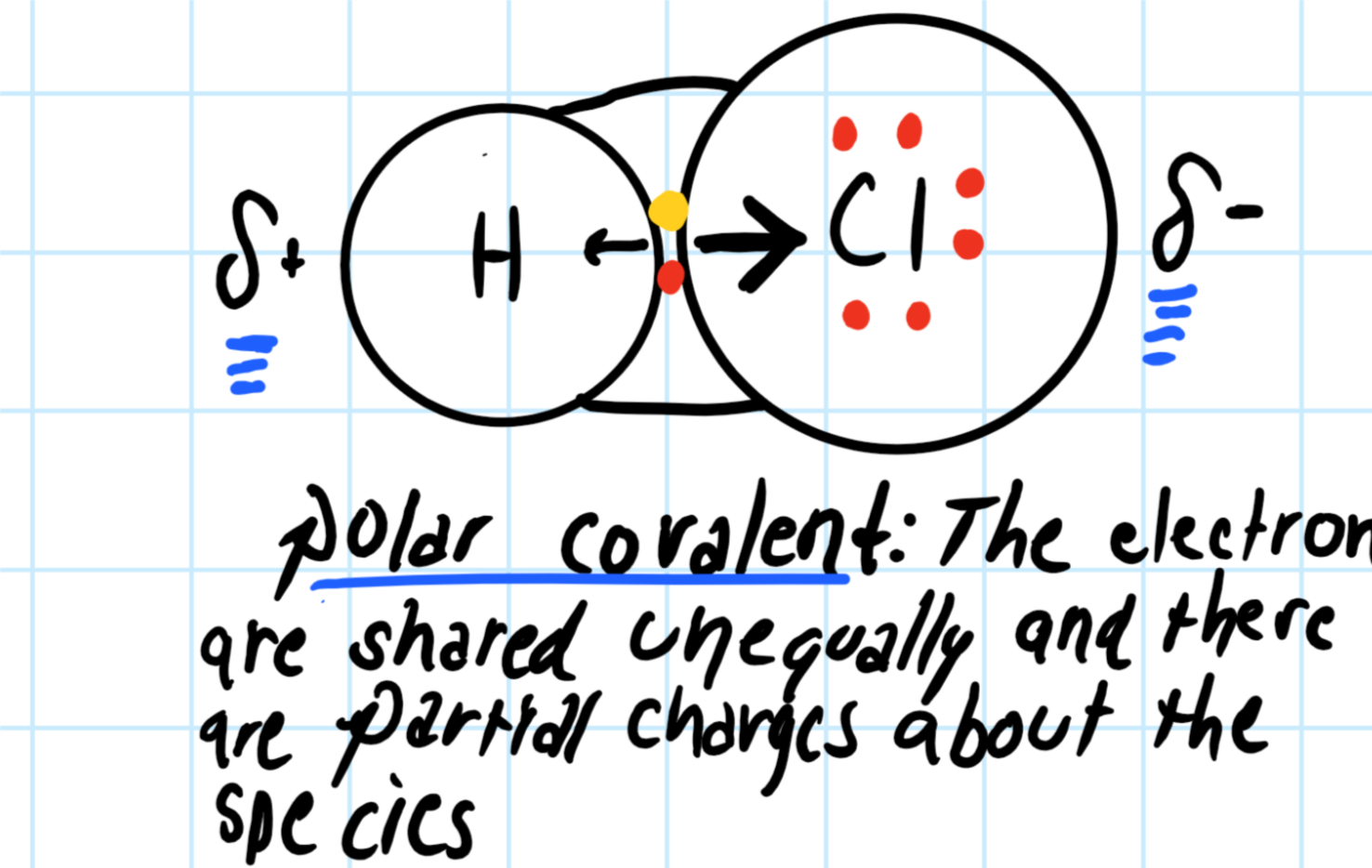
Lewis dot diagram of hydrogen. Lewis structure of Hydrogen sulfide (H2S) contains two S-H single bonds around sulfur atom. Also, there are two lone pairs around sulfur atom. Concept of number of total valence electrons of sulfur and hydrogen atoms are used to draw lewis structure of H2S. NF3 lewis structure has 3 fluorine and 1 nitrogen atom connected with three single bonds. This structure is very similar to NCl3 and NH3. There is a total of 10 lone pairs and 3 bonded pairs present in the NF3 lewis structure. Let’s see how to draw lewis dot structure for NF3 with easy steps. Simple steps for drawing the NF3 lewis dot ... Hydrogen Lewis Structure. Here are a number of highest rated Hydrogen Lewis Structure pictures upon internet. We identified it from reliable source. Its submitted by management in the best field. We put up with this kind of Hydrogen Lewis Structure graphic could possibly be the most trending topic later than we ration it in google gain or facebook. The lesser the electronegativity of an atom more is the ability to share electrons, hence, the least electronegative always takes the central place in the lewis diagram. In the case of the CH3CN molecule, hydrogen always goes outside as it can share only a maximum of two electrons, from carbon and nitrogen atom, carbon is the less ...
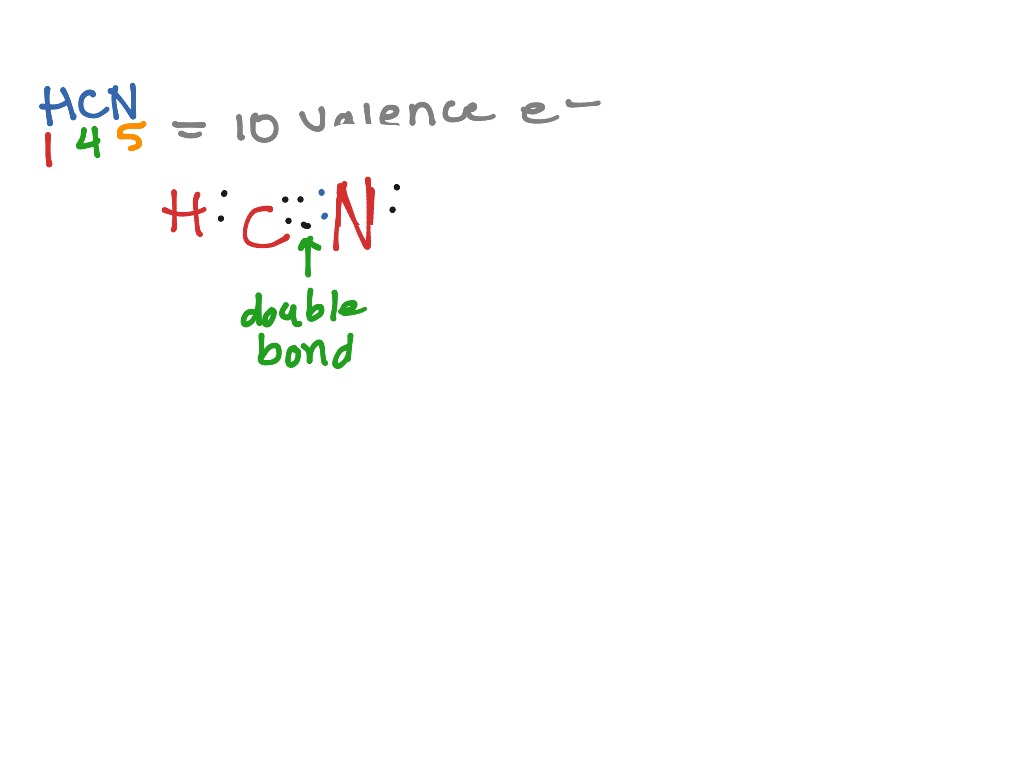
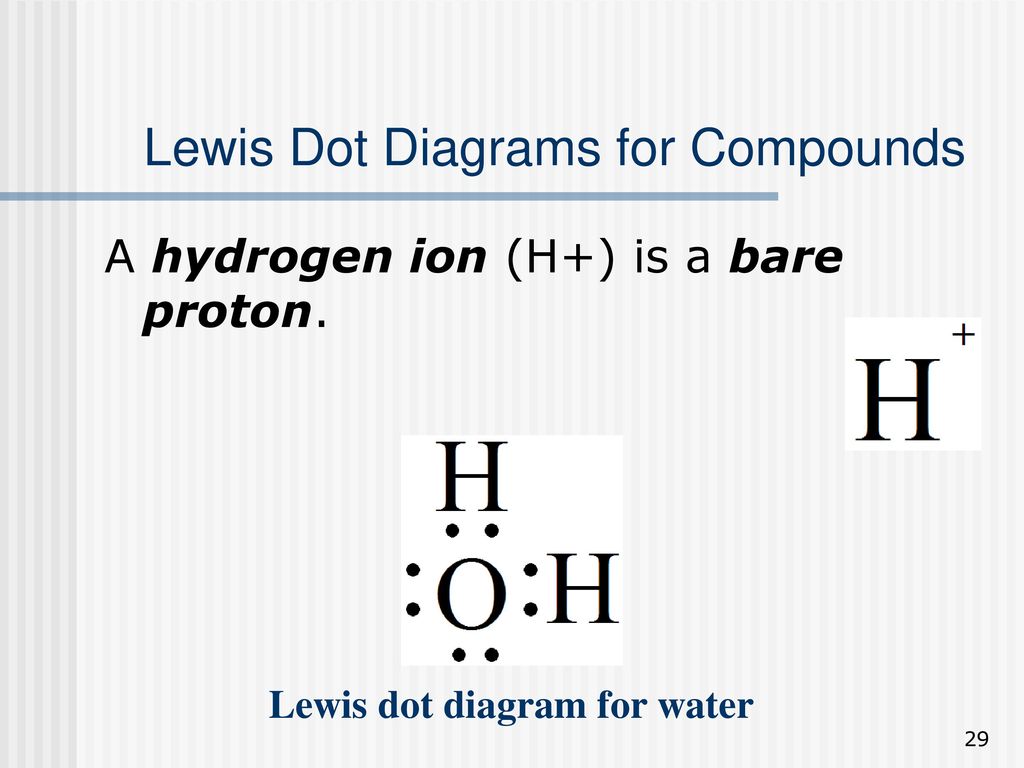
The first shell (n=1) can have only 2 electrons, so that shell is filled in helium, the first noble gas. In the periodic table, the elements are placed in "periods" and arranged left to right in the order of filling of electrons in the outer shell. So hydrogen and helium complete the first period. There are 2 valence electrons from the hydrogen atoms, and 6 valence electrons from the oxygen atom..... And thus we have to distribute 8 electrons in the Lewis dot diagram. Of course, the electronic geometry is tetrahedral that leads to /_H-O-H = 104.5^@. The molecular geometry is bent. Note that I distinguish between electronic geometry, and molecular geometry. The Lewis Structure (Lewis Dot Diagram) for HCN.1. Count electrons2. Put least electronegative atom in centre3. Put one electron pair in each bond4. Fill out... The Lewis dot structure for water shows the electron from hydrogen and an electron from oxygen being shared in a covalent bond. The other four valence electrons in oxygen are in pairs at the bottom. The lines are a short-hand version of the two dots representing the covalent bonds.
A step-by-step explanation of how to draw the H2O2 Lewis Dot Structure (Hydrogen peroxide). Note that the H2O2 Lewis structure is frequently used on tests a... A step-by-step explanation of how to draw the H2 Lewis Dot Structure (Hydrogen gas).For the H2 structure use the periodic table to find the total number of v... Jan 28, 2022 · SF6 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram SF6 or sulfur hexafluoride is an inorganic and one of the most stable gases that are known in chemistry. This gas has more density than air. 2 days ago · Draw the lewis diagram: The Geometrical Structure of the H2O molecule. The bond angle among hydrogen-oxygen-hydrogen atoms (H-O-H) is 104.5°. From this, it can be understood that the geometrical structure of a single H2O molecule is bent.
A step-by-step explanation of how to draw the HPO4 2- Lewis Dot Structure (Hydrogen phosphate ion).For the HPO4 2- structure use the periodic table to find t...
the Lewis dot diagram is a table used for the elements, and it shows you how many valence electrons there are. ;)
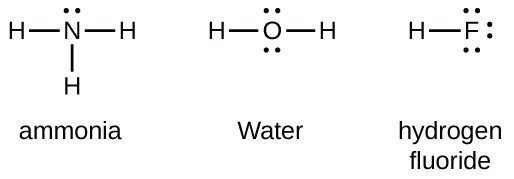
(15) Lewis Diagrams Obj. 15. From the name of a molecular chemical, determine the Lewis (electron dot) diagram for it.. In these cases, you know that you are dealing with nonmetal atoms bonded together with covalent bonding and that (some of) the valence electrons of the atoms are shared between the atoms.
Lewis Dot Structures Objectives: 1. Draw Lewis structures for atoms, ions and simple molecules. 2. Use Lewis structures as a guide to construct three-dimensional models of small molecules. 3. Determine the electron and molecular geometry of the produced molecules. Background: Scientists often create models to represent either a physical or ...
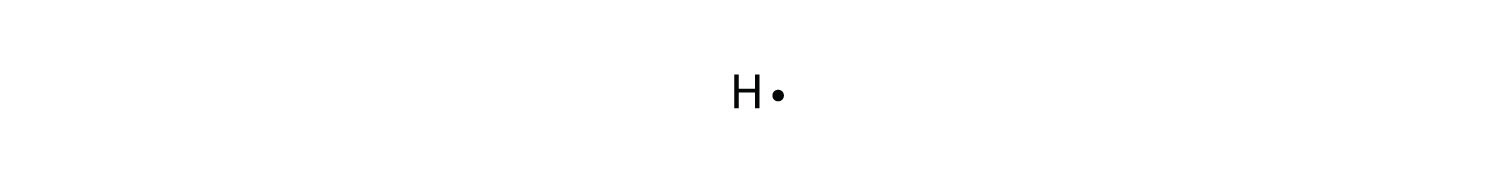
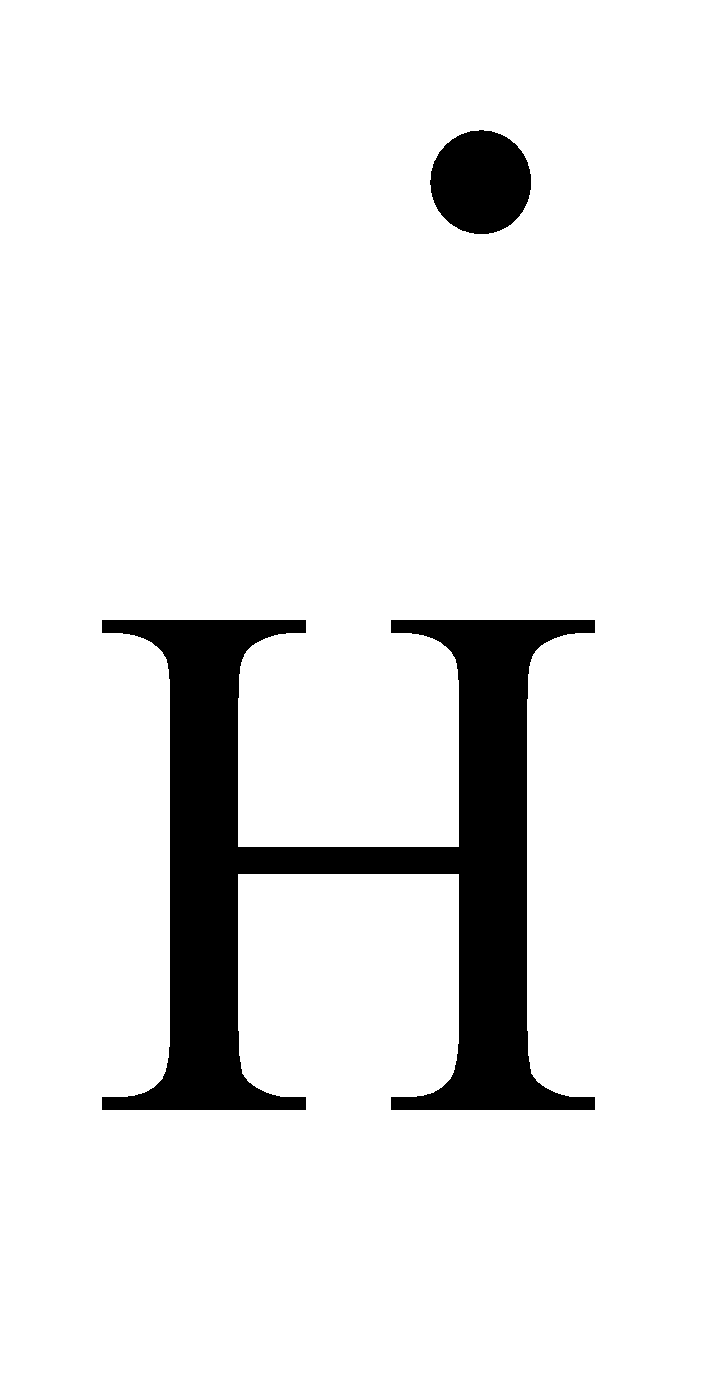
This type of Lewis dot structure is represented by an atomic symbol and a series of dots. See the following examples for how to draw Lewis dot structures for common atoms involved in covalent bonding. Example 1. Draw the Lewis Dot Structure for the Hydrogen atom. Since Hydrogen is in Group I it has one (1) valence electron in its shell. Example 2.
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
Jul 15, 2021 · Contributors; Lewis used simple diagrams (now called Lewis diagrams) to keep track of how many electrons were present in the outermost, or valence, shell of a given atom.The kernel of the atom, i.e., the nucleus together with the inner electrons, is represented by the chemical symbol, and only the valence electrons are drawn as dots surrounding the chemical …
Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place them around the elements symbol. Element Atomic # Atomic Mass Protons Neutrons Electrons Lewis Dot Carbon 6 12 6 6 6 l Hydrogen 1 1 1 0 1 H Lithium 3 7 3 4 3 Li
For atoms with partially filled d or f subshells, these electrons are typically omitted from Lewis electron dot diagrams. For example, the electron dot diagram for iron (valence shell configuration 4s 2 3d 6) is as follows:. Elements in the same column of the periodic table have similar Lewis electron dot diagrams because they have the same valence shell electron configuration.
Carbon Monoxide Co Lewis Structure Chemistry Worksheets Lewis Carbon Monoxide . Glow In The Dark Lewis Dot Diagrams Senior Chemistry Quimica Organica Ligacao Quimica Ensino De Quimica . Lithium Electron Configuration Electron Configuration Electrons Diagram Design . Lewis Dot Structure Covalent Bonding Chemistry Worksheets Electron Configuration













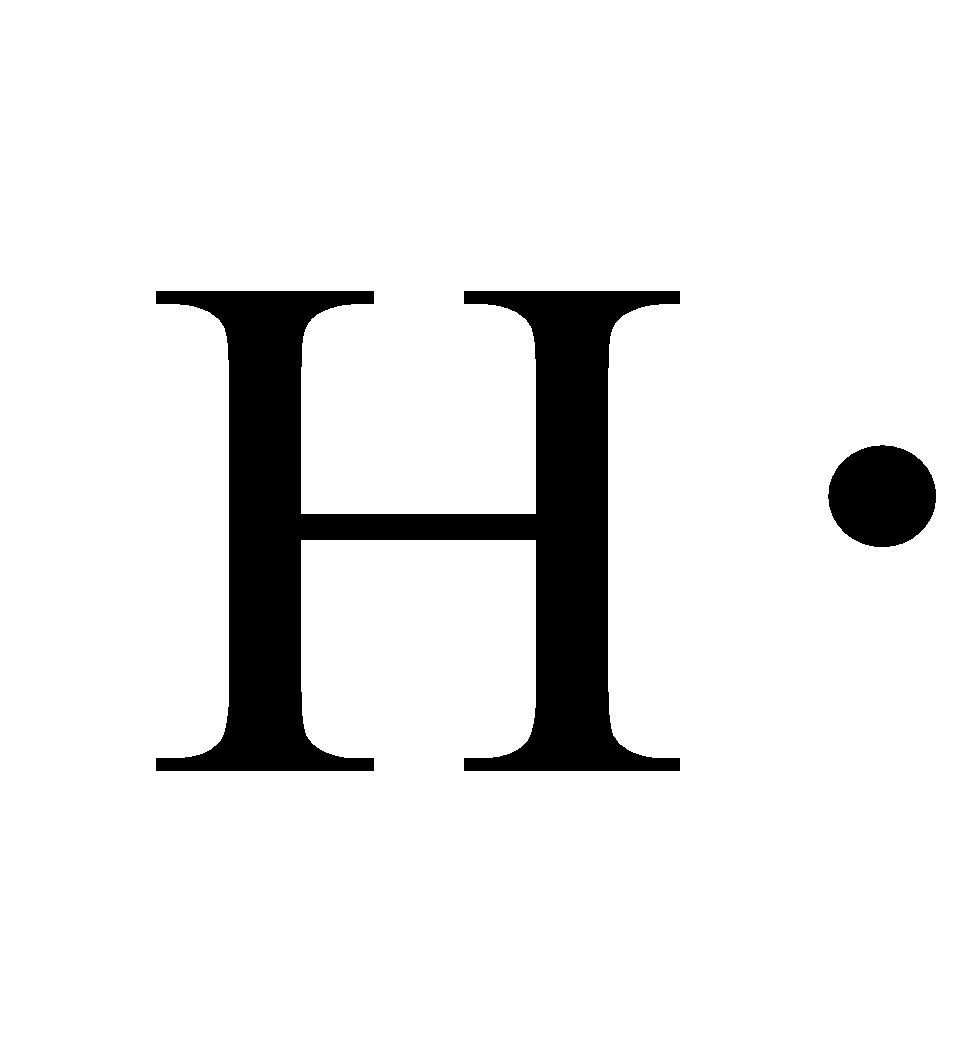













Comments
Post a Comment