43 n2 dot diagram
Describe the electron dot diagram system of representing structure. Draw electron dot diagrams for ... nitrogen, 1 s 2 2 s 2 2 p 3, 5 valence electrons.Nitrogen: 1 s 2 2 s 2 2 p 3Neon: 1 s 2 2 s 2 2 p 6Lithium: 1 s 2 2 s 1Beryllium: 1 s 2 2 s 2 &-1. ffte bond in a diatomic nitrogen molecule (N2) best described as (1) polar (2) polar double covalent @nonpolar triple covalent (4) polar ionic]$. wt i.h electron dot diagram represents H2? (1) H. H (3):H.H: @; H:n Look at the electron dot formula shown below. The attraction of X for the bondinq electrons,,^,,culd be greatest ,rvhen X ,"pr ...
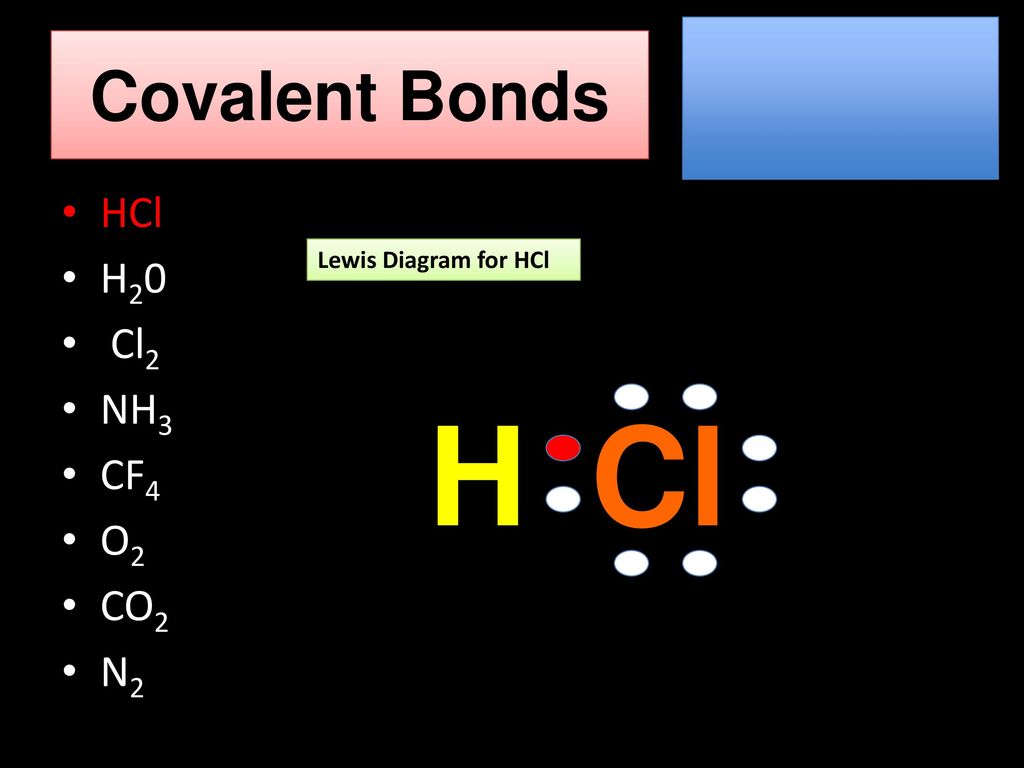
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
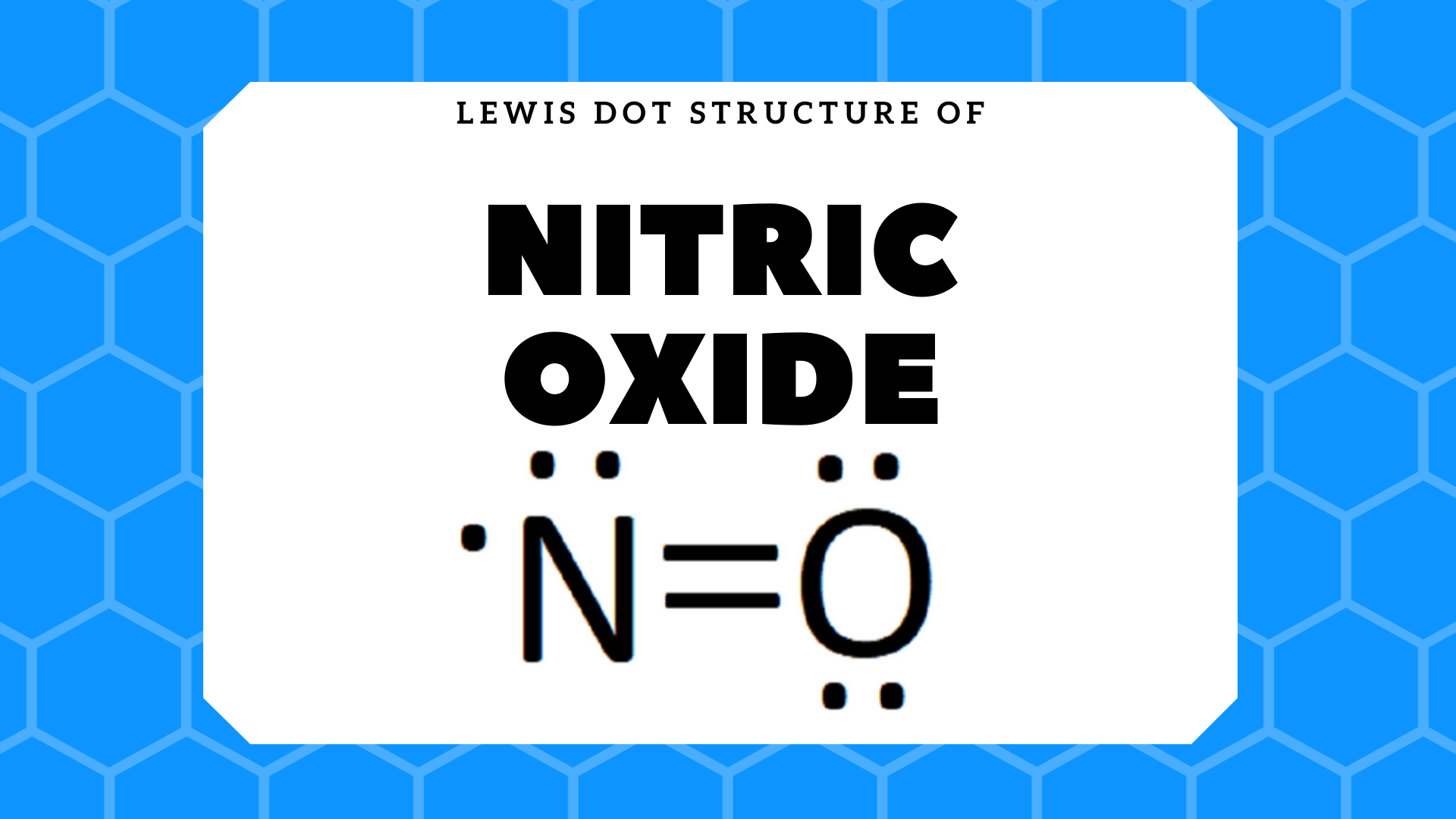
N2 dot diagram
Lewis dot structure of n2 molecule Let's take a look at the Lewis of and N2 structure. Atomic nitrogen has 5 valence electrons and 4 orbital valence (2s, 2 px, 2py and 2pcs). In the Lewis structure there is a triple link between nitrogen atoms and a pair of non-binding electrons on each. This is consistent with the physical properties of N2. College. answer. answer. answered. Based on the Lewis electron-dot diagrams of n2 and n2h4, compare the length of the nitrogen-to-nitrogen bond in n2 with the length of the nitrogen-to-nitrogen bond in n2h4. 2. Answer (1 of 2): In exactly the same way as you'd do it for any simple, covalently bonded compound. First you need to know the valency of each element. Then you need to draw a stick model with bonds between the atoms, so that each atom's valency is satisfied (so N has three bonds coming out of i...
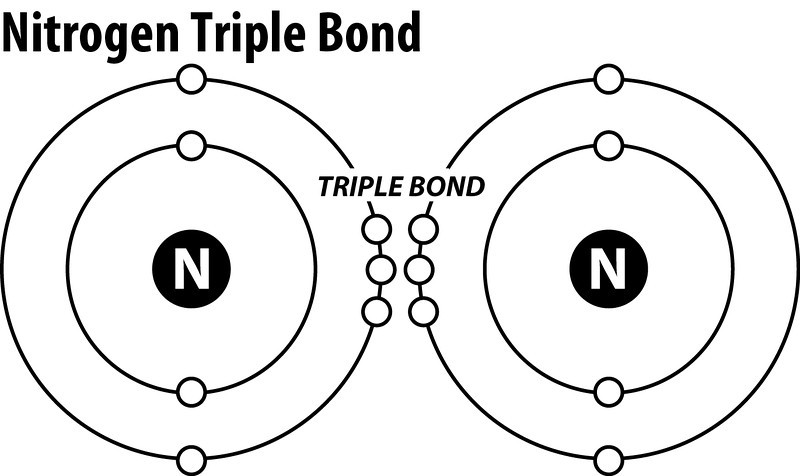
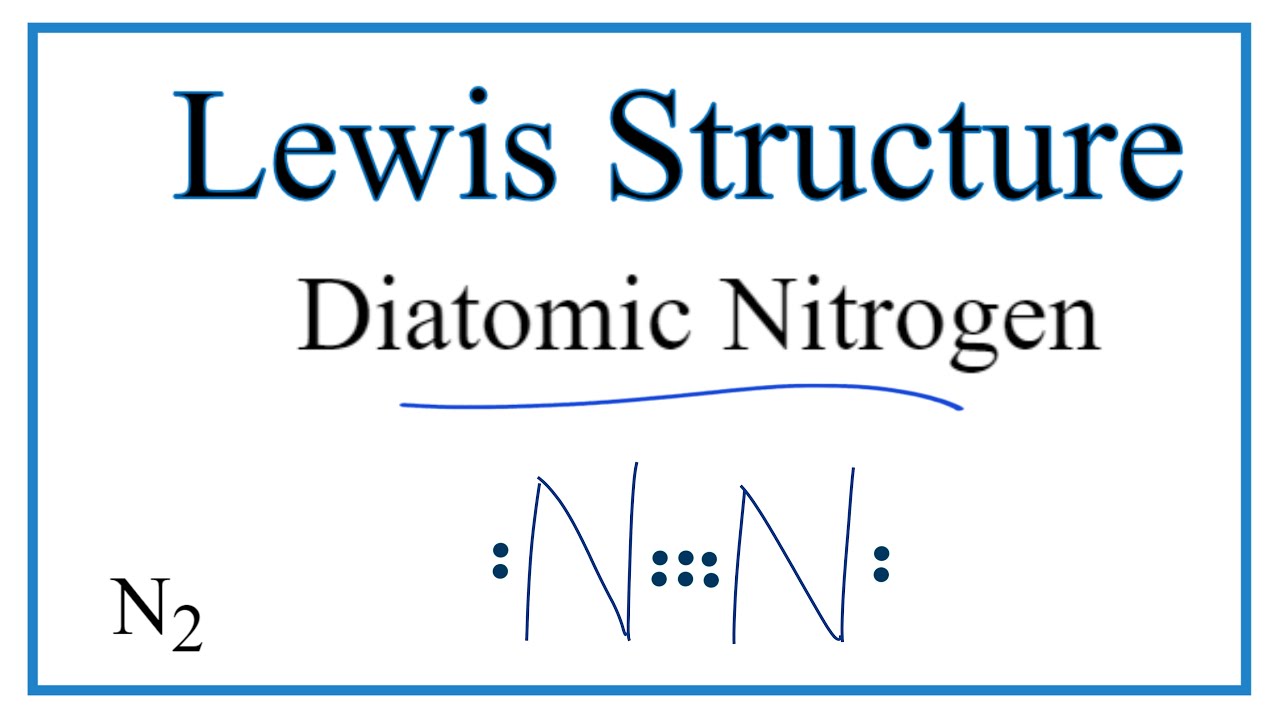
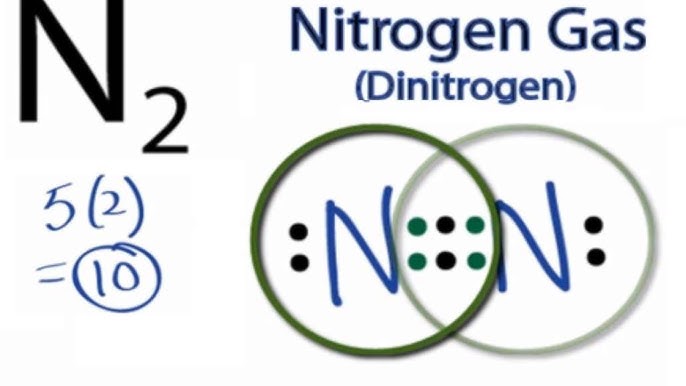
N2 dot diagram. May 9, 2017 — So nitrogen has 7 electrons, if we show the electron configuration it's 1S2, 2S2, 2P3, the P sub shell has 3 orbitals with 6 potential electrons and so needs to ...2 answers · 0 votes: In exactly the same way as you’d do it for any simple, covalently bonded compound. First ...What is the Lewis Structure for N2 (nitrogen gas)?3 answersDec 31, 2015What is the Lewis Dot Structure for Mg3N2?4 answersOct 22, 2018More results from www.quora.com Draw the Electron-dot Structure of N2 and State the Type of Bonding. - Science. ... Draw the electron-dot structure of N 2 and state the type of bonding. Advertisement Remove all ads. Solution Show Solution. Electron-dot structure of N 2 is. In N 2, one nitrogen atom shares its three electrons with another nitrogen atom to form a covalent bond. Nitrogen (N 2) Molecule Lewis Structure. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Lewis structure of N 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There are many things to learn when we draw N 2 lewis structure.. N 2 lewis structure. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. The diagram is drastically out of scale, as the relative size of the nucleus compared to the surrounding electrons is usually comparable to a pea in a stadium. N2 Properties The N 2 Lewis structure shows two nitrogen atoms bonded in the same way to each other. It's perfectly symmetric. Generally, small symmetric molecules are nonpolar.
Lewis Dot Diagram For N2 19.11.2018 7 Comments Plucked this image from google. There are 3 dots (electrons) in the middle for each Nitrogen atom because Nitrogen molecules form triple. The Lewis Structure for N2 looks easy at first. The problem is that there aren't enough valence electons to give both Nitrogen atoms an octet. You'll need to use . n2 lewis structure bond dot molecular nitrogen does molecule valence diatomic electrons triple lone pair nonmetals occurs each atom exists . electron nitrogen dot ionic bonding diagram ne write compounds chapter electrons valence symbol ppt powerpoint presentation slideserve . Transcript: For the N2 Lewis structure, we have five valence electrons for Nitrogen--it's in group 5 or 15 on the periodic table. We have two Nitrogens. Multiply those together, we have a total of 10 valence electrons for the N2 Lewis structure. We'll put the two Nitrogens next to each other, and then we'll put two valence electrons between them to form a chemical bond. If you are talking about the Lewis Dot Diagram then N2 would have 5 dots around each of the letter N's, so that there would be 6 dots total (triple bond) between the two N's and a pair of dots ...
3. Explain N2 dot structure in simplest form. N2 dot structure would comprise of two atoms of Nitrogen(N) atoms. There is a triple bond between both nitrogen atoms. Each N is surrounded by two dots which are called lone pairs of electrons. It is a diatomic nonpolar molecule with bond angles of 180 degrees. Nitrogen (N2) is a commonly tested Lewis structure due to its importance on Earth (about 78% of the Earth's atomsphere is N2). It also is a good example of a ...May 15, 2013 · Uploaded by Wayne Breslyn Nitrogen /induced CNS depression/ ("rapture of the deep" or "the martini effect") results from a direct toxic effect of high nitrogen pressure on nerve conduction and produces effects similar to alcohol intoxication. Complex reasoning, decision-making ability, motor function, and manual dexterity decrease. Click here👆to get an answer to your question ️ Draw the electron dot structure for oxygen molecule. Solve Study Textbooks. Join / Login >> Class 10 >> Chemistry >> Carbon and its compounds >> Covalent bonding in carbon compounds >> Draw the electron dot structure for oxyg. Question .
Answer (1 of 3): As nitrogen is in fifth group in periodic table therefore it will have five electrons in the valance shell in which three electrons are unpaired because it needs three electrons to complete its outermost shell. Therefore in case of N2, each nitrogen atom will share three electron...
How to Draw the Lewis Structure of N2 - with explanation!Check me out: http://www.chemistnate.com
N2 dot structure would comprise of two atoms of Nitrogen(N) atoms. There is a triple bond between both nitrogen atoms. Each N is surrounded by two dots which are called lone pairs of electrons. It is a diatomic nonpolar molecule with bond angles of 180 degrees. 6. Explain o2 lewis structure in simplest form
What is the Lewis dot diagram for nitrogen? Each N is surrounded by two dots and three sticks or lines, representing another 6 electrons in the N2 triple bond. So each N is surrounded by 8 total valence electrons, giving it an octet and making it stable.
Now, distribute valence electrons around the atoms of N2. Since you have 2 atoms of Nitrogen, assign the valence electrons using dots in a diagram to each atom-like 5 dots around each atom. Use symbol N to represent the atom. Both the atoms have the same electronegativity, there will be no central atom in the structure.
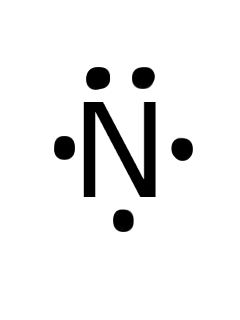
In order to come up with this answer, you first need to know the number of valence electrons for Nitrogen. Since #N# is a member of the Group 5A (based on the periodic table), the number of electrons in its outermost shell must be 5.. Here is the electron dot structure for a single #N# atom:. The total number of valence electrons between the two #N# atoms is #10 e^-#.
A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Nitrogen Gas - Diatomic Nitrogen).For the N2 structure use the periodic table to find t...
Nitrogen is a triple bonded molecule. Since Nitrogen belongs to the diatomic molecule in the VA family, on the periodic tables, which means that ...Dec 20, 2020 · Uploaded by Wayne Breslyn
Answered: Draw the Lewis Dot Structure for N2.… | bartleby. Draw the Lewis Dot Structure for N2. What type of bond keeps this molecule together (single, double, or triple)? Is the bond polar or nonpolar and why?
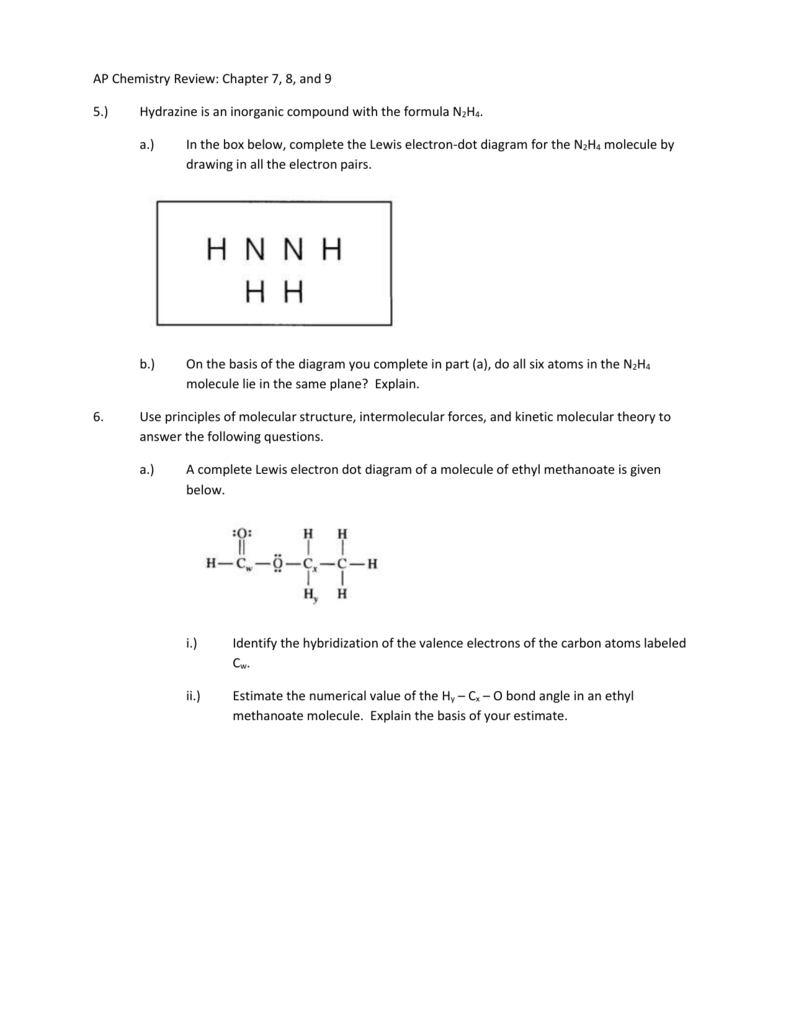
N2 can react with H2 to form the compound N2H4. (f) The Lewis electron-dot diagram of N2H4 is shown below. (i) Based on the Lewis electron-dot diagrams of N2 and N2H4, compare the length of the nitrogen-to-nitrogen bond in N2 with the length of the nitrogen-to-nitrogen bond in N2H4.
Show the formation of N2 by electron dot structure and orbital structure diagram 2 See answers Advertisement Advertisement jenishanto2004 jenishanto2004 Answer: For the N2 Lewis structure, we have five valence electrons for Nitrogen--it's in group 5 or 15 on the periodic table. We have two Nitrogens. ...
Chemistry questions and answers. (a) In the box below, draw the complete Lewis electron-dot diagram of N2 . (b) Based on the Lewis electron-dot diagram that you drew, is the N2 molecule polar? Explain. B I : E 0 / 10000 Word Limit The following graph shows the potential energy of two nitrogen atoms versus the distance between their nuclei.
Answer (1 of 2): In exactly the same way as you'd do it for any simple, covalently bonded compound. First you need to know the valency of each element. Then you need to draw a stick model with bonds between the atoms, so that each atom's valency is satisfied (so N has three bonds coming out of i...
College. answer. answer. answered. Based on the Lewis electron-dot diagrams of n2 and n2h4, compare the length of the nitrogen-to-nitrogen bond in n2 with the length of the nitrogen-to-nitrogen bond in n2h4. 2.
Lewis dot structure of n2 molecule Let's take a look at the Lewis of and N2 structure. Atomic nitrogen has 5 valence electrons and 4 orbital valence (2s, 2 px, 2py and 2pcs). In the Lewis structure there is a triple link between nitrogen atoms and a pair of non-binding electrons on each. This is consistent with the physical properties of N2.











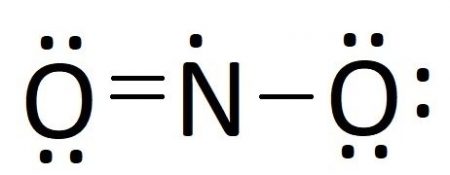














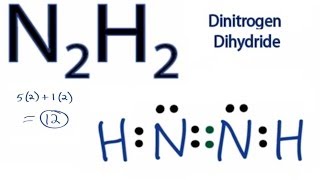

![Draw the electron dot structure of Nitrogen molecule [N = 7]](https://haygot.s3.amazonaws.com/questions/1890007_1909574_ans_16e2a124f2974b5694de1a9f3c97eebd.png)
Comments
Post a Comment