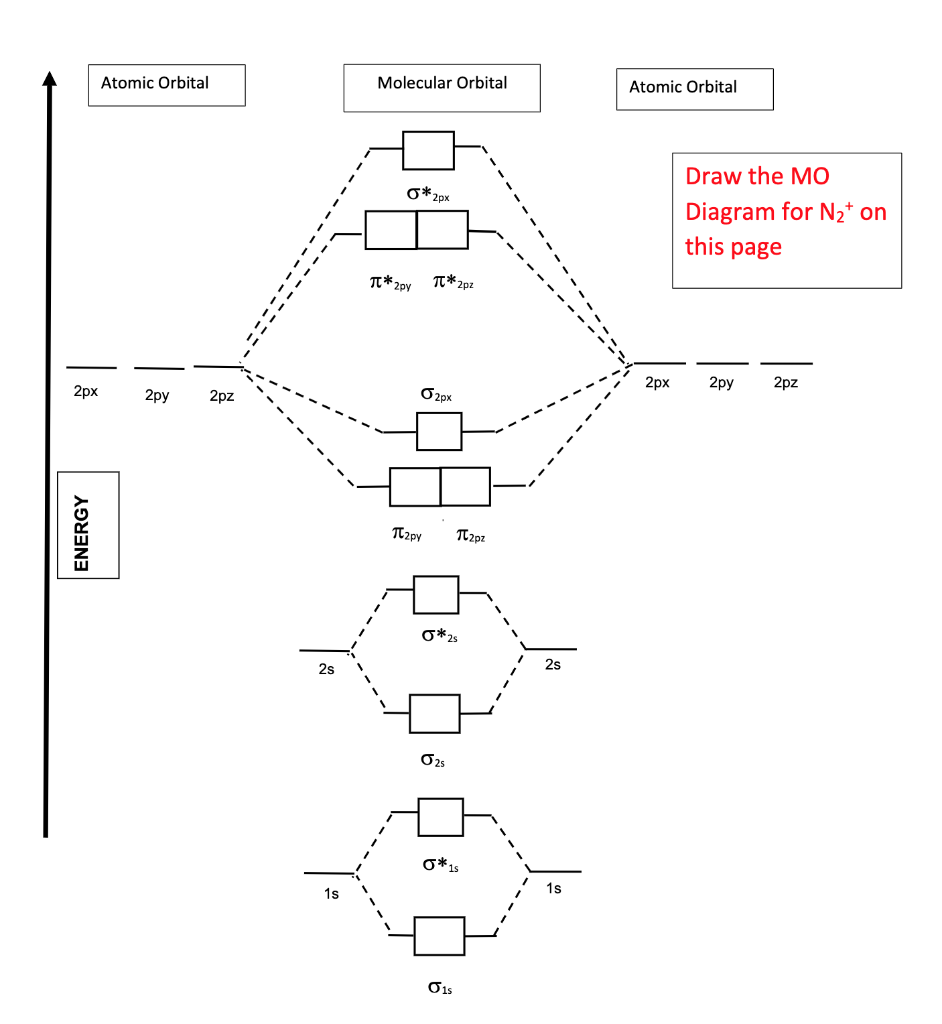
43 molecular orbital diagram for n2
A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row hey! I have a question: how do i draw a molecular orbital diagram for SO2? i only found examples for diatomic diagrams and im not sure how to do it if i have more then two atoms in the molecule.
Shouldn’t you count the valence electrons for Be (which is 2) and subtract 1 because of the + sign? For O2, N2, NO, F2, etc, you count the number of valence electrons instead of the atomic number. Why is it that for Be, though, you look at the atomic number instead of the number of valence electrons it has? I apologize if this is a stupid question, but I appreciate any clarification on this

Molecular orbital diagram for n2
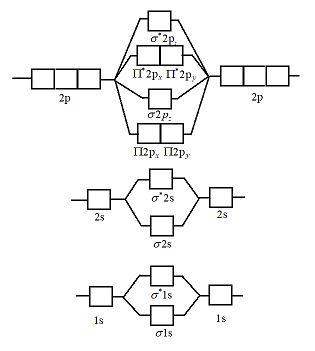
The molecular orbital theory explains how there are no unpaired electrons in the bonds between the two N atoms. The 1s and 2s molecular orbitals are completely filled There are no electrons in the any of the 2p anti-bonding orbitals. Seeing a molecular orbital diagram for N2 will clarify what i mean. A molecular orbital diagram without electrons is like an apartment building without people. So let's pick the simplest possible molecule to apply to this system: butadiene. 8. Populating The Molecular Orbitals Of Butadiene With Electrons. Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).Bond...
Molecular orbital diagram for n2. Jan 31, 2022 · Molecular Orbitals diagram of Phosphorus Trifluoride (PF3) molecule. The molecular orbital diagram helps with determining how chemical bond formation is taking place. Also, it helps with figuring out how mixing and overlapping have taken place to produce four new hybrid orbitals. Molecular orbital diagram for nitrogen gas (N2) Use aufbau and Hund to fill with 10 valence electrons You get sigma2s(2) ... Molecular Orbital Diagram for Nitrogen Gas ( 1 ion) (N2( )). Fill from the bottom up, with 9 valence electrons total. Bonding Order ... The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. Figure 9. The molecular orbital energy diagram predicts that H2 will be a stable molecule with lower energy than the separated atoms. When the phase diagram for a substance has a solid-liquid phase boundary line that has a negative slope (leans to the left), the substance _____. a. can go from solid to liquid, within a small temperature range, via the application of pressure
https://i.imgur.com/GgRlFtK.jpg (Not homework) I am trying to improve by using past papers. Can someone explain how to solve these 3 questions? molecular orbital diagram for O2. number of elections in the sigma*2p molecular orbital is. which response lists all the following diatomic molecules and ions that have at least one unpaired electron (Be2, B2, B2+, C2, N2, N2+). • The following slide illustrates the relative energies of the molecular orbitals compared to the original atomic orbitals. • Because the energy of the two electrons is lower than the energy of the individual atoms, the molecule is stable. Figure 9.26: (a) The molecular orbital energy-level diagram for the... Construct the molecular orbital diagram for dichlorine. Orbitals and molecular representation representations of molecules. DINITROGEN N2 Textbook orbitals.
James E. Brady The Molecular Nature of Matter (6th Edition) Copia. Download. James E. Brady The Molecular Nature of Matter (6th Edition) Copia The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. Figure 8.35 The molecular orbital energy diagram predicts that H2 will be a stable molecule with lower energy than the separated atoms. What happens to the molecular orbital diagram when a metal-ligand complex is oxidized? Oxidation removes an electron, e.g. you go from d8 metal to d7 metal. As consequence the antibonding orbital has an unpaired electron making the complex less stable (weaker M-L bond, since less pi-backdonation), but how does it change the gap between the metal MO and LUMO of ligand, as well as the gap between the metal MO and HOMO of the ligand? Molecular orbital : A molecule in which all the electrons are paired, is called diamagnetic. | Online Chemistry tutorial IIT, CBSE Chemistry, ICSE Chemistry, engineering and medical chemistry entrance exams Molecular orbital diagram of C2 molecule : Number of electrons in C2 molecule = 12.
Molecular Orbital (MO) Theory is the final theory pertaining to the bonding between molecules. The proper notation is that molecular orbitals are written just by the kind of bond that the orbital creates. An anti-bonding orbital is written as the bond with the star superscripted onto it.
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
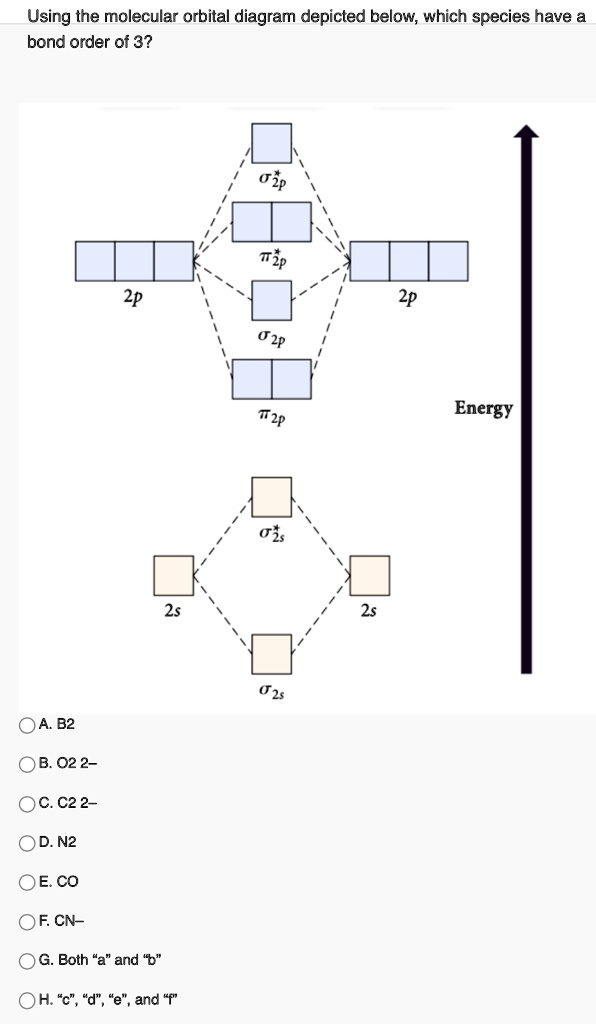
TOP: Homonuclear Diatomic Molecules 32. Draw the molecular orbital diagram for O 2 . The number of unpaired electrons is _ ___. The total numbers of electrons in the 2p molecular orbitals of B 2 is ____. OBJ: Determine the MO diagram given the molecule formula.
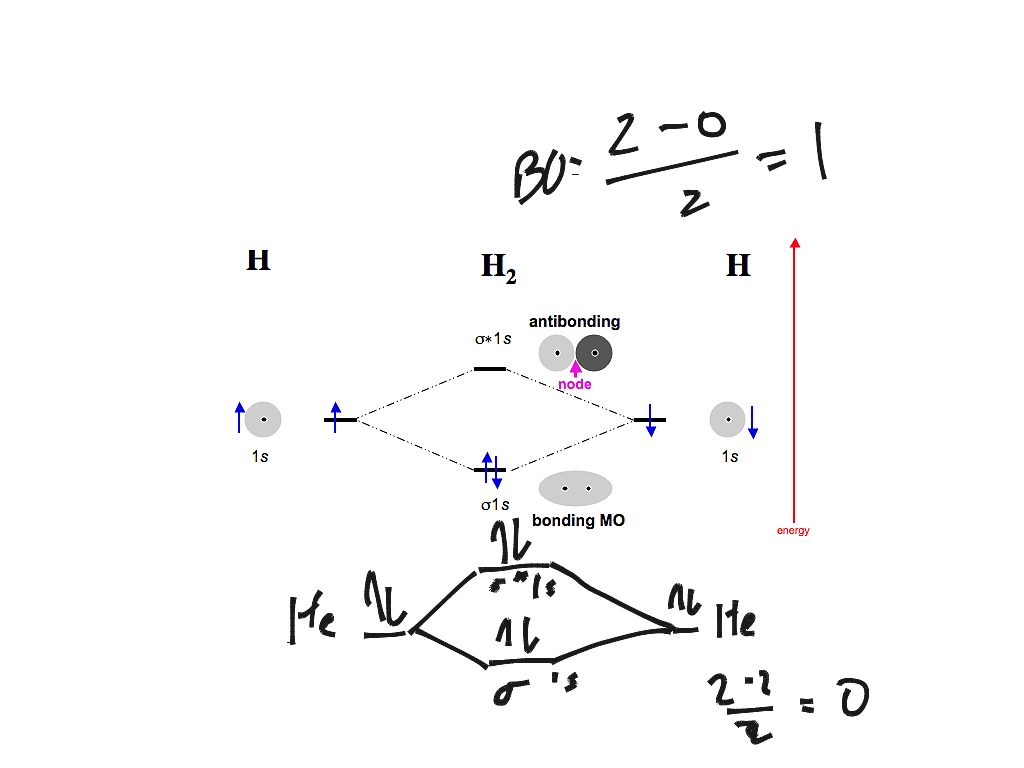
Drawn the molecular orbital diagram and write the bond orrder, magnetic property of `N_(2)` molecule and `N_(2)^(o+)` ion ? Draw the molecular orbital energy level diagram for `H_2` and discuss its stability, bond order and magnetic character.
The Journal of the American Chemical Society (JACS), founded in 1879, is the flagship journal of the American Chemical Society and the world’s preeminent journal in all of chemistry and interfacing areas of science. This periodical is devoted to the publication of fundamental research papers and publishes approximately 19,000 pages of Articles, Communications, and Perspectives a year ...
Draw the molecular orbital energy level diagram of N2 molecules. > Explain the molecular orbital structure, bond order, stability and magnetic behavior of Hydrogen molecule on the basis of molecular orbital theory.
I have been taught that the MO diagram is different for molecules with 14 or less electrons than the one used for molecules with 15 or more electrons. All this is simply because the primitive molecular orbital theory does not explain the things, rather it rationalizes them (for differences between...
[At 27oC, five identical rigid 2.0 L vessels are filled with N2(g) and sealed. Four of the five vessels also contain a 0.050 mol sample of NaHCO3(s), NaBr(s), Cu(s), or I2(s), as shown in the diagram above. The volume taken up by the solids is negligible, and the initial pressure of N2(g) in each vessel is 720 mm Hg.
The molecular orbital diagram for an O2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions Experiments have shown that O2 and F2 are best described by the model in the figure above, but B2, C2, and N2 are best described by a model that...
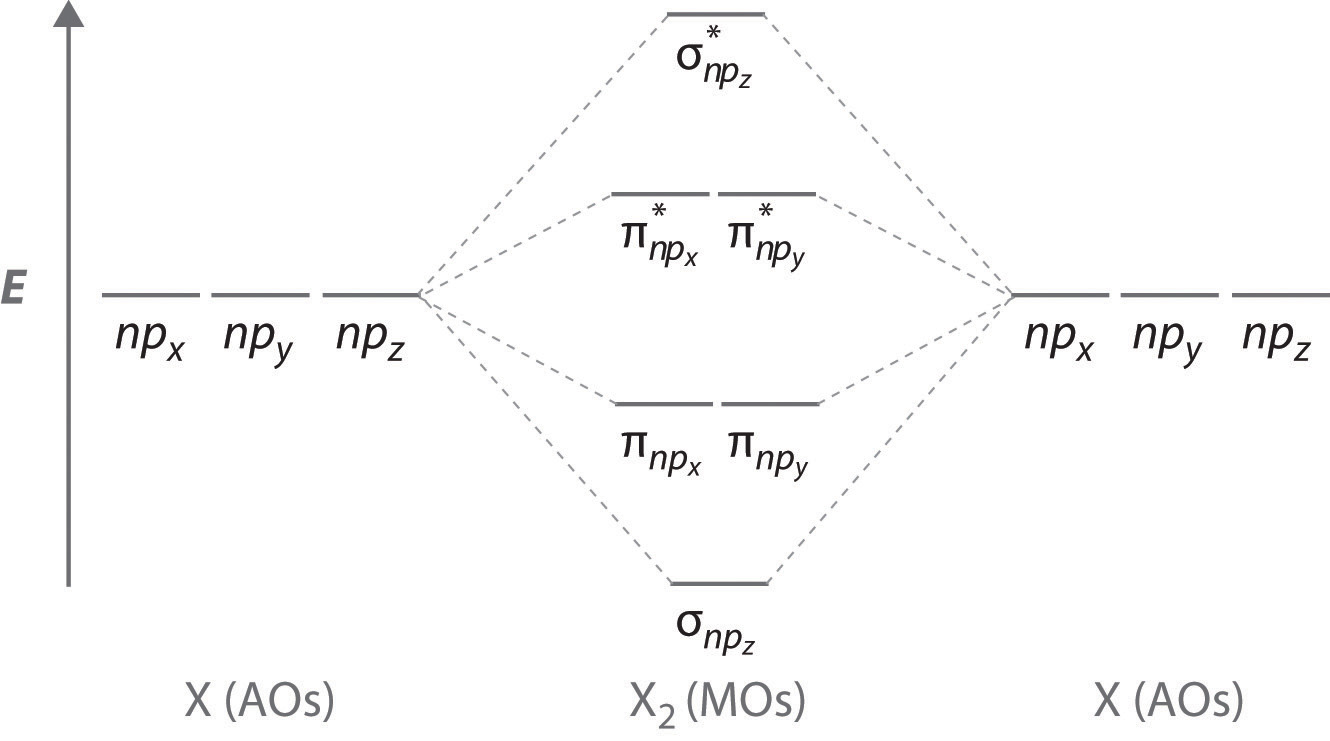
Molecular orbital diagrams are diagrams of MO energy levels, shown as short horizontal lines in the center. Atomic orbitals (AO) energy levels are shown for comparison. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels.
The diagram shows how the molecular orbitals in lithium hydride can be related to the atomic orbitals of the parent atoms. This is smaller than the 945 kJ bond energy of N2— not surprising, considering that oxygen has two electrons in an antibonding orbital, compared to nitrogen's one.
Let me explain the molecular orbital diagram of N2 using its diagram. one atom of nitrogen has 7 electrons so a N2 molecule will have 14 electrons. If we compare such diagrams for the diatomic molecules on the Second Period (Li₂, Be₂, B₂, C₂, N₂, O₂, and F₂), the resulting pattern looks like this
I need to construct the molecular orbital diagram for the hypothetical species Li4, which has the following geometrical arrangement: https://preview.redd.it/npsjre5pch571.png?width=197&format=png&auto=webp&s=c2a7948c2efa04a975bee1db722838fae7482456 The first step is to identify the point symmetry group. In this particular case, we consider that there is only one axis of rotation of order four (actually, other symmetry elements can be observed, but this is a previous consi...
The molecular orbital energy diagram predicts that He2 will not be a stable molecule, since it has equal numbers of bonding and antibonding Eight possible homonuclear diatomic molecules might be formed by the atoms of the second period of the periodic table: Li2, Be2, B2, C2, N2, O2, F2, and Ne2.
I've been getting the hang of creating MO diagrams and I understand the very basics. My problem is in the 2p orbital's bonding section where sometimes the pi 2p section is lower energy than the sigma 2p section (i.e MO diagram for B2 diatomic molecule). I understand that the lower energy must be filled in first and so my question is, how do I know if the pi 2p is lower energy than sigma 2p?
One of the most essential words in the science of chemistry is bond order. The chemical bonds that exist between two atmos are determined by determining the bond order. Bond order can also be used to get a sense of a molecule's stability, bond energy, bond length, and thermal stability. However, determining bond order via MOT or by drawing molecular orbitals takes time. So, in this article, I've attempted to offer a novel technique to calculating the bond order using applied mathematics and ...
I’m a little confused on the connection between a molecules molecular orbital diagram and it’s individual atomic hybridization. Can anyone help me? Thank you
Sep 17, 2018 · [A more rigorous way to treat it is from a molecular orbital perspective – a weak bond results in a low-energy LUMO, and therefore a lower energetic barrier to attack by nucleophiles]. However strange it might look, the benzyne intermediate explains all of these important observations, and more.
Figure 9-2 Molecular orbital (MO) diagram for the combination of the 1s atomic orbitals on two identical atoms (at the left) to form two MOs. Experimental thermodynamic data show that the N2 molecule is stable, is diamagnetic, and has a very high bond energy, 946 kJ/mol.
Figure 4.10.1: Molecular Orbital Energy-Level Diagrams for Homonuclear Diatomic Molecules.(a) For F2, with 14 valence electrons (7 from To obtain the molecular orbital energy-level diagram for O2, we need to place 12 valence electrons (6 from each O atom) in the energy-level diagram shown in...
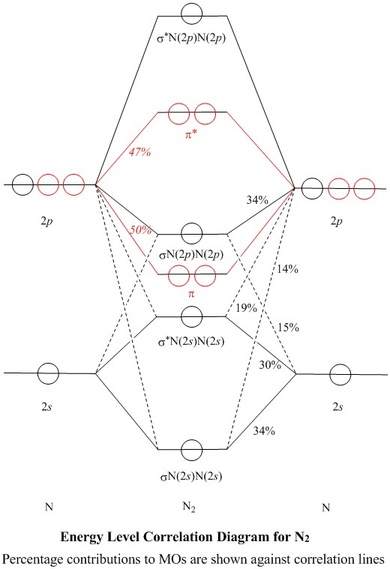
Molecular Orbitals for N2. Jmol models of calculated wavefunctions. To view a model, click on a molecular orbital in the energy level correlation Most of these squares are shown as percentages against the correlation lines of the Energy Level Correlation Diagram. All of the valence shell NAO...
Draw the molecular orbital diagram for N2- ion, and calculate the bond order. Indicate if it is diamagnetic or paramagnetic.
- MO diagrams for Inorganic complexes. 2. Lecture 1 Lecture 2. • It is a waste of both the lecturers and students time if the tutorial to ends up being a lecture covering questions. 5. An introduction to Molecular Orbital Theory.
I’ve been tasked with drawing rhe MO diagram for Sulfure Oxide and I’m not sure about the energies of the relatove orbitals. Since Oxygen is more electronegative I expect the 2s and 2p orbitals to have much lower energy than the 3s and 3p orbitals sulfur has. But the energy difference would be really high then. So I’m not sure what 2 orbitals combine to form the sigma 3s or sigma* 3s orbital. The difference in energy kevels confuses me as every example I’ve done has the same orbitals (2s,2p’s) c...
The molecular orbital diagram representing this order of energy levels is shown in fig. For example, homonuclear diatomic molecules of second row elements like Li2, Be2, B2 , C2, N2 , the σ 2pz MOs is higher in energy than π 2px and π 2py MOs.
Molecular orbital diagram for nitrogen gas (N2) Use aufbau and Hund to fill with 10 valence electrons You get sigma2s(2) ... In this example problem, we show how to fill a molecular orbital diagram for a diatomic molecule and use molecular bond theory ...
Answer : According to the molecular orbital theory, the general molecular orbital configuration will be, As there are 7 electrons present in nitrogen. The bond order of is, 3. The molecular orbital diagram of are shown below.
I know how to draw MO diagrams for certain bonds like NF and CN-, but I don't know how to draw an MO diagram for a bond between a first period element and a second period element.
CBSE Class 11 Chemistry Notes are Best ever notes prepared by our awesome team members. We have spend more that 2 years to prepare these Class 11 chemistry notes.After analyzing our notes in deep, we have uploaded our notes on the website.
Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).Bond...
A molecular orbital diagram without electrons is like an apartment building without people. So let's pick the simplest possible molecule to apply to this system: butadiene. 8. Populating The Molecular Orbitals Of Butadiene With Electrons.
The molecular orbital theory explains how there are no unpaired electrons in the bonds between the two N atoms. The 1s and 2s molecular orbitals are completely filled There are no electrons in the any of the 2p anti-bonding orbitals. Seeing a molecular orbital diagram for N2 will clarify what i mean.










![Solved] Given the Molecular Orbital Diagram for Dinitrogen ...](https://d2lvgg3v3hfg70.cloudfront.net/TB2288/11ea7a3a_9ef5_0197_a82d_e3348e31dff5_TB2288_00.jpg)



.png)











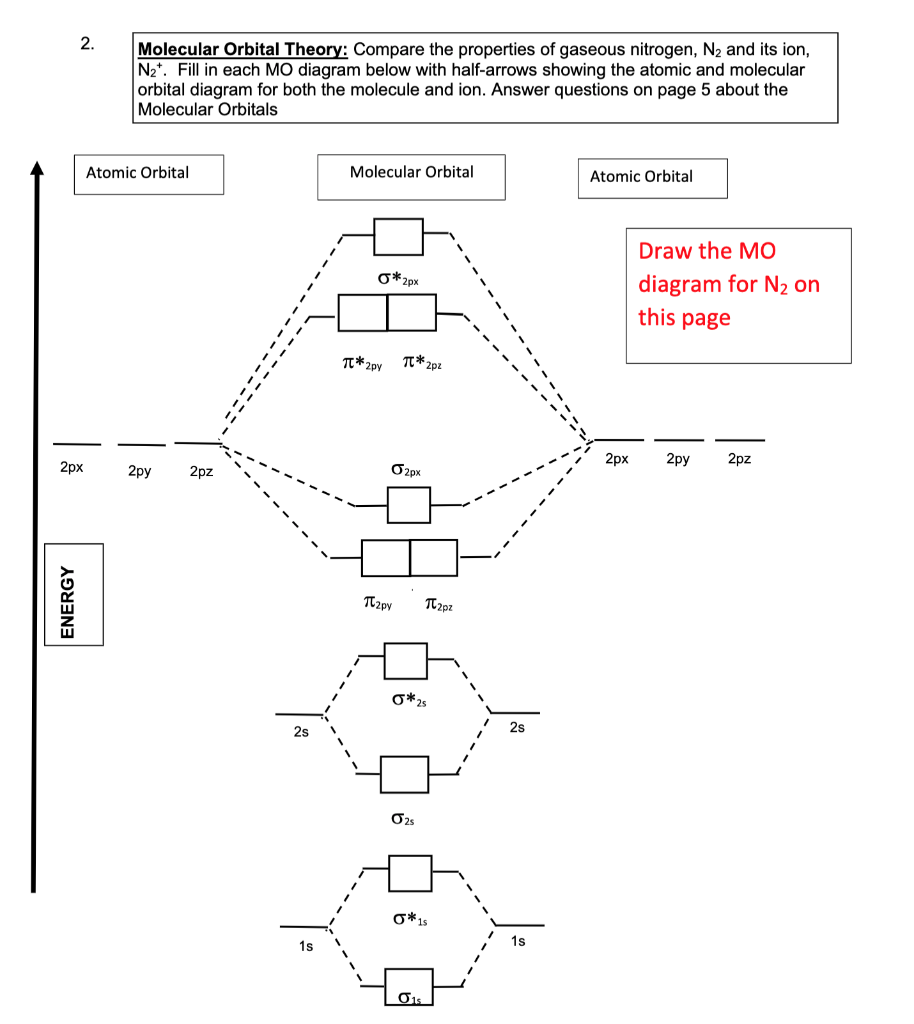

Comments
Post a Comment