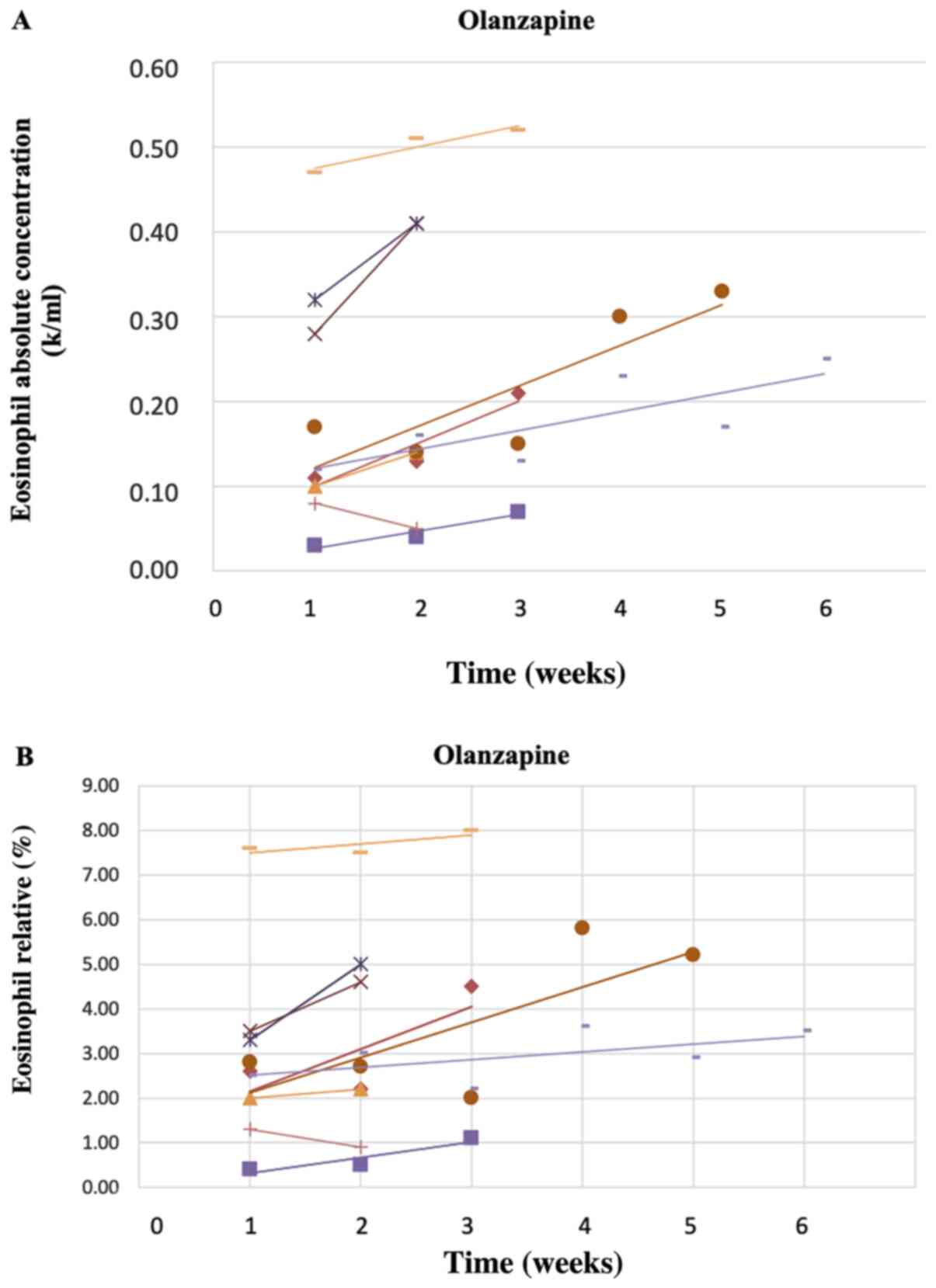
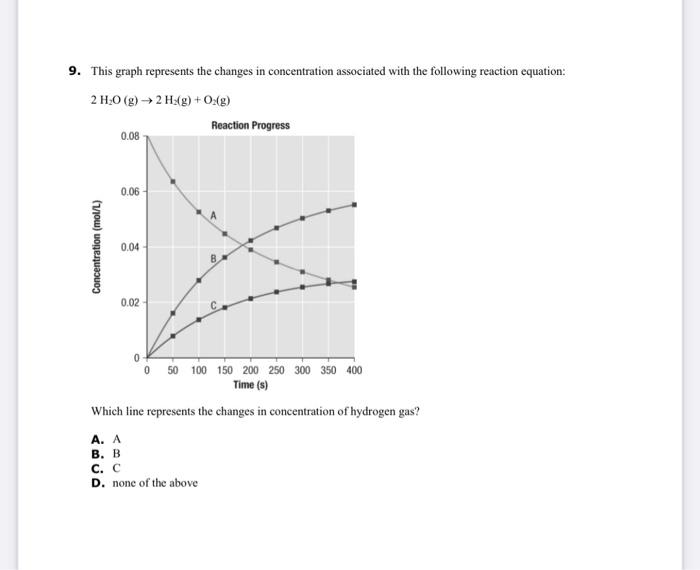
39 in the diagram the black line represents the concentration
In the diagram, the black line represents the concentration of... Answer:B. At the end of the reaction, both product and reactants are of a constant concentration.Explanation:Option A and C are similar as they both. Which statement best describes the reaction rate? A. The product maintains an constant concentration in the first half of the reaction. In The Diagram The Black Line Represents The Concentration Time o the reactants maintain an constant concentration in the first half of the reaction o the product maintains an constant concentration...
In The Diagram The Black Line Represents The Concentration The area with the highest concentration of. 4enzyme production 17the diagram below represents a cell and several molecules.
In the diagram the black line represents the concentration
Ellingham Diagrams (all content) The diagram is essentially a graph representing the thermodynamic driving force for a particular reaction to occur, across a range of temperatures. The Gibbs free energy, G, of a system can be described as the energy in the system available to do work. It is one of the most useful state functions... Frontiers | Linking Chlorophyll Concentration and Wind Patterns Using... Using satellite chlorophyll concentration from GlobColour together with QuikSCAT and ASCAT winds, we examine the relationship between shelf (shallower than the 150 m isobath) chlorophyll concentration and wind patterns in the Central and Northern CCS. 8.3 Le Chatelier's principle | Chemical equilibrium | Siyavula Le Chatelier's Principle helps to predict what effect a change in temperature, concentration or pressure will have on the position of the equilibrium in a chemical reaction. So if the concentration of one (or more) of the reactants or products is increased the equilibrium will shift to decrease the concentration.
In the diagram the black line represents the concentration. Liquid-Solid Phase Diagrams: Salt Solutions - Chemistry LibreTexts This line represents the effect of increasing amounts of salt on the freezing point of water. Using Phase Diagrams. The phase diagram is used to find out what happens if you cool salt solution of a particular concentration. We need to look at three separate cases. literarydevices.net › symbolismExamples and Definition of Symbolism - Literary Devices Definition of Symbolism. Symbolism is a literary device that refers to the use of symbols in a literary work. A symbol is something that stands for or suggests something else; it represents something beyond literal meaning. Fluency Focus Academy - IELTS: IELTS Writing Task 1:-- The... The diagram further reveals that, the groundwater flows from East to West directions passing near to the salt water interface reaching to its destination sea and The heaviest concentration of nitrogen is below the landfills followed closely by industries while it is less near the sea. Concentration - density. In the diagram, the black line represents the concentration of... C. The reactants maintain an constant concentration in the first half of the reaction. D. At the end of the reaction, both product and reactants are of an equal concentration. I think its A. Please correct if I am wrong.
PDF lecture 14 If the concentration (activity) of C+ in the analyte solution changes, the voltage measured between the two reference electrodes also changes. The reference electrode at the right side of the line diagram is the straight Ag | AgCl electrode at the centre of the electrode (see next slide). 3. Are human activities causing climate change? | Australian Academy... Human activities have increased greenhouse gas concentrations in the atmosphere. Atmospheric concentrations of carbon dioxide (CO2), methane and nitrous oxide began to rise around two hundred years ago, after changing little since the end of the last ice age thousands of years earlier. practicalmaintenance.netThe Iron-Iron Carbide Equilibrium Diagram This line is called the lower-critical-temperature line on the hypoeutectoid side and is labeled A 1. The A 1 line is the eutectoid-temperature line and is the lowest temperature at which f.c.c. iron can exist under equilibrium conditions. Just above A 1 line, the microstructure consists of approximately 25 percent austenite and 75 percent ... Phase Change Reactions Precipitation-Dissolution of Inorganic Species... The light colored lines represent the concentrations of individual Al species. The heavy black line represents the total concentration of all Al species, i.e 22 Log activity dissolved Si Species This plot illustrates the principles discussed in the previous slide. The light red lines show the concentrations...
Electrochemical Properties of the Platinum Metals | Johnson Matthey... The lines representing the equilibrium conditions of each reaction are obtained by substituting the relevant values of E° or K in equations (2), (3) or (4) for given concentrations of reactant and product (if these are in the liquid phase) and plotting the corresponding values of E and pH. Explainer: The high-emissions 'RCP8.5' global warming scenario The Representative Concentration Pathways (RCPs). Projecting future climate change involves assessing a number of different uncertainties. Some of these relate to the climate system, such as how sensitive the climate might be to increased concentrations of greenhouse gas in the atmosphere. Climate Change Indicators: Atmospheric Concentrations of... | US EPA Each line represents a different data source. Data source: Compilation of eight underlying This figure shows concentrations of nitrous oxide in the atmosphere from hundreds of thousands of For example, sulfate particles can reflect sunlight away from the Earth, while black carbon aerosols (soot)... Expressing Concentration of Solutions represents the amount of solute dissolved in a unit amount of solvent or of solution, and. can be expressed in a variety of ways (qualitatively and quantitatively). Microscopic view of a concentrated solution of liquid Br2 dissolved in liquid water. Semi-Quantitative Expressions of Concentration.
en.ecgpedia.org › wiki › BasicsBasics - ECGpedia Jan 14, 2021 · A ladder diagram is a diagram to explain arrhythmias. The figure shows a simple ladder diagram for normal sinus rhythm, followed by av-nodal extrasystole. The origin of impulse formation (sinus node for the first two beats and AV junction for the third beat) and the conduction in the heart are shown.
6.1 The Budget Line - Principles of Microeconomics Recreate budget lines after prices and income changes. The Budget Line. To understand how households make decisions, economists look at what consumers can afford. Notice that areas in the green zone are not necessarily more optimal than points along the budget line.
Solved: In The Diagram, The Black Line Represents... | Chegg.com Transcribed Image Text from this Question. In the diagram, the black line represents the concentration of a reactant and the green line represents the concentration Which of the following statements best describes the reaction rate?
Помогите пожалуйста с тестами по английскому языку which type of... Помогите пожалуйста с тестами по английскому языку Which type of money are paid to professional people such architects and lawyers? Ответы а) currency б)fees в bonus г salary 2.The money paid for the use of house or flat Ответы a bills бrent в bonus г coins 3Choose the synonym of social security...
Calculated DOS of catechol-TiO 2 systems (the black line represents... Download scientific diagram | Calculated DOS of catechol-TiO 2 systems line represents the total DOS; the filled cyan, red and green lines represent the PDOS of catechol in W 6+ doped, pure and Zn 2+ By fine-tuning the W-doping concentration to 0.25%, aqueous DSCs produced a significant...
Metals | Free Full-Text | The Eh-pH Diagram and Its Advances | HTML Feature Papers represent the most advanced research with significant potential for high impact in the field. The diagrams are divided into areas, each of which represents a locally-predominant species. The axis can represent the solubility of stable solids, ligand concentration or temperature.
PDF Microsoft PowerPoint - TERNARY-PHDIAGS-1.PPT Diagrams that represent the equilibrium between the various phases that are formed between three components, as a function of temperature. Normally, pressure is not a viable variable in ternary phase diagram construction, and is therefore held constant at 1 atm.
PDF PowerPoint Presentation | Binary phase diagram U' represents composition of liquid phase and V' represents composition of solid phase as intersection U meets liquidus line and V meets solidus line. Ø These points are specific in the sense that they occur only at that particular conditions of concentration, temperature, pressure etc.
Beer's Law - Theoretical Principles The diagram below shows a beam of monochromatic radiation of radiant power P0, directed at a sample solution. We will express this measurement in centimetres. c is the concentration of the compound in solution, expressed in mol L-1. Note that the Law is not obeyed at high concentrations.
› biotechnology › wasteWaste and Waste Water Treatment (With Diagram) Soluble sulphide in water reacts with hydrogen ion concentration to form HS or H 2 S. The toxicity of sulphide is derived from the H 2 S rather than the sulphide ion. The lethal concentration of sulphide for fish life is reported to be between 0.032 and 0.355 mg/l.
Diagram based multiple choice Flashcards | Quizlet Identify the black circled part of the diagram. Note the one that is least likely to be on the test is the color one. Which graph best represents the effect of temperature on the rate of photosynthesis of a plant? The diagram below shows the light independent stage of photosynthesis (the Calvin Cycle).
Isoquant and Isocost Lines (With Diagram) | Economics An isocost line is a locus of points showing the alternative combinations of factors that can be In fact, every point on a given isocost line represents the same total cost. All the isocost lines in the diagram have the same slope because the relative prices of labour and capital are the same.
en.wikipedia.org › wiki › Hertzsprung–Russell_diagramHertzsprung–Russell diagram - Wikipedia The Hertzsprung–Russell diagram, abbreviated as H–R diagram, HR diagram or HRD, is a scatter plot of stars showing the relationship between the stars' absolute magnitudes or luminosities versus their stellar classifications or effective temperatures.
› ternary-phase-diagramTernary Phase Diagram - an overview | ScienceDirect Topics A point on the diagram represents a composition that is specified in terms of mole fraction or weight fraction. The point, (0.3, 0.4, 0.3) is at the center of the small triangle in the diagram and is located by following the red diagonal 60° line at red 0.3 and the horizontal line at blue 0.4 or any combination of two of the coordinates (A, B, C).
Defect concentration diagram - Wikipedia The defect concentration diagram (also problem concentration diagram) is a graphical tool that is useful in analyzing the causes of the product or part defects. It is a drawing of the product (or other item of interest), with all relevant views displayed...
PDF Antti | Fig. 2. Default values for the Eh - pH - diagram. The lines in the diagrams represent the Eh-pH-conditions where the content of the adjacent species is the same in the equilibrium state. The Labels and Lines sheets are in the programs internal use and it is not necessary to make any modifications to these sheets.
en.wikipedia.org › wiki › BlackBlack - Wikipedia In Paganism, black represents dignity, force, stability, and protection. The color is often used to banish and release negative energies, or binding. An athame is a ceremonial blade often having a black handle, which is used in some forms of witchcraft. Sports
Concentration Units | Boundless Chemistry Compared to molar concentration or mass concentration, the preparation of a solution of a given molality is easy because it requires only a good scale; both solvent and solute are massed, rather than measured by The mole fraction is used very frequently in the construction of phase diagrams.
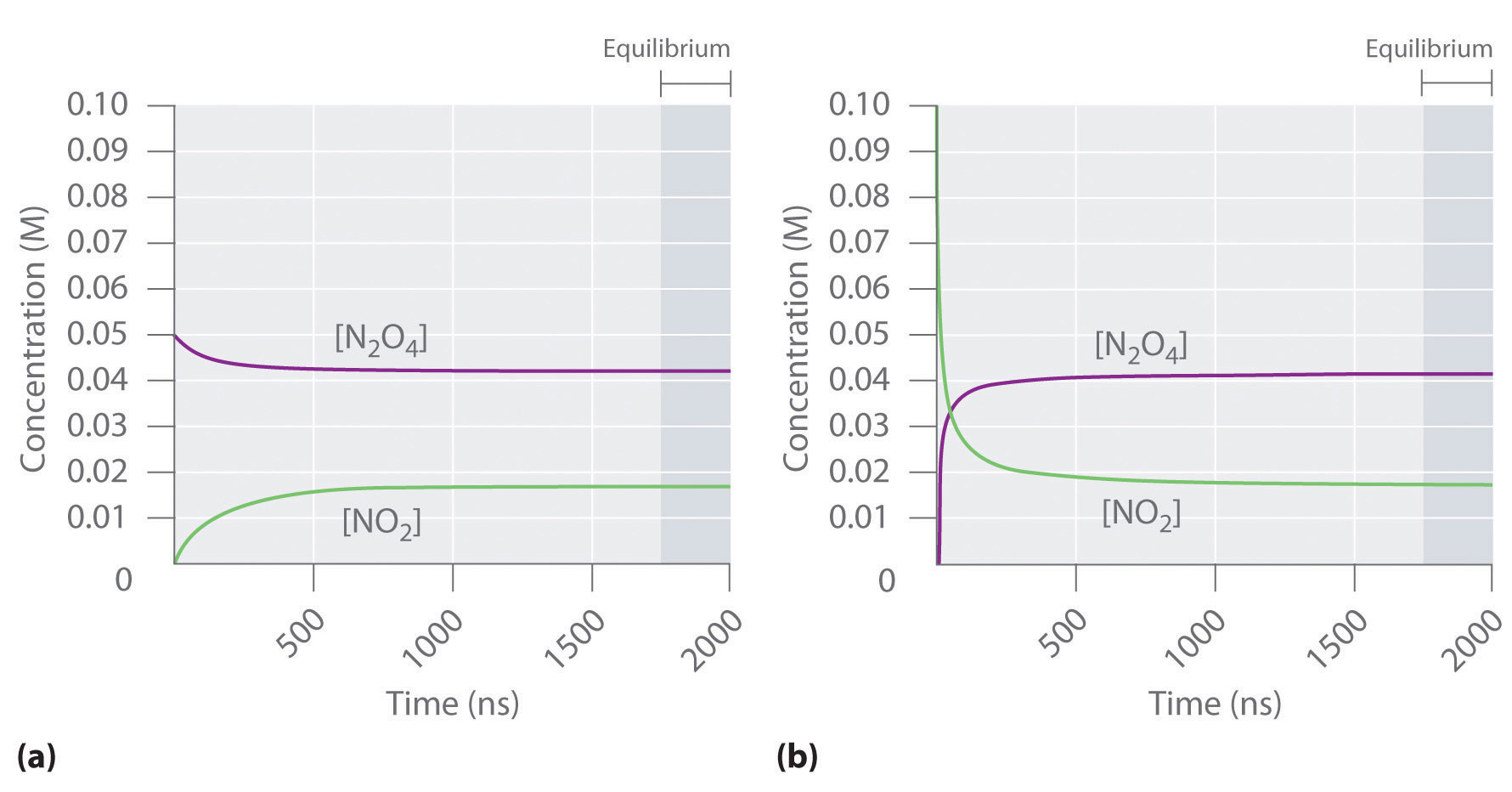
8.3 Le Chatelier's principle | Chemical equilibrium | Siyavula Le Chatelier's Principle helps to predict what effect a change in temperature, concentration or pressure will have on the position of the equilibrium in a chemical reaction. So if the concentration of one (or more) of the reactants or products is increased the equilibrium will shift to decrease the concentration.
Frontiers | Linking Chlorophyll Concentration and Wind Patterns Using... Using satellite chlorophyll concentration from GlobColour together with QuikSCAT and ASCAT winds, we examine the relationship between shelf (shallower than the 150 m isobath) chlorophyll concentration and wind patterns in the Central and Northern CCS.
Ellingham Diagrams (all content) The diagram is essentially a graph representing the thermodynamic driving force for a particular reaction to occur, across a range of temperatures. The Gibbs free energy, G, of a system can be described as the energy in the system available to do work. It is one of the most useful state functions...
















Comments
Post a Comment