40 endothermic potential energy diagram
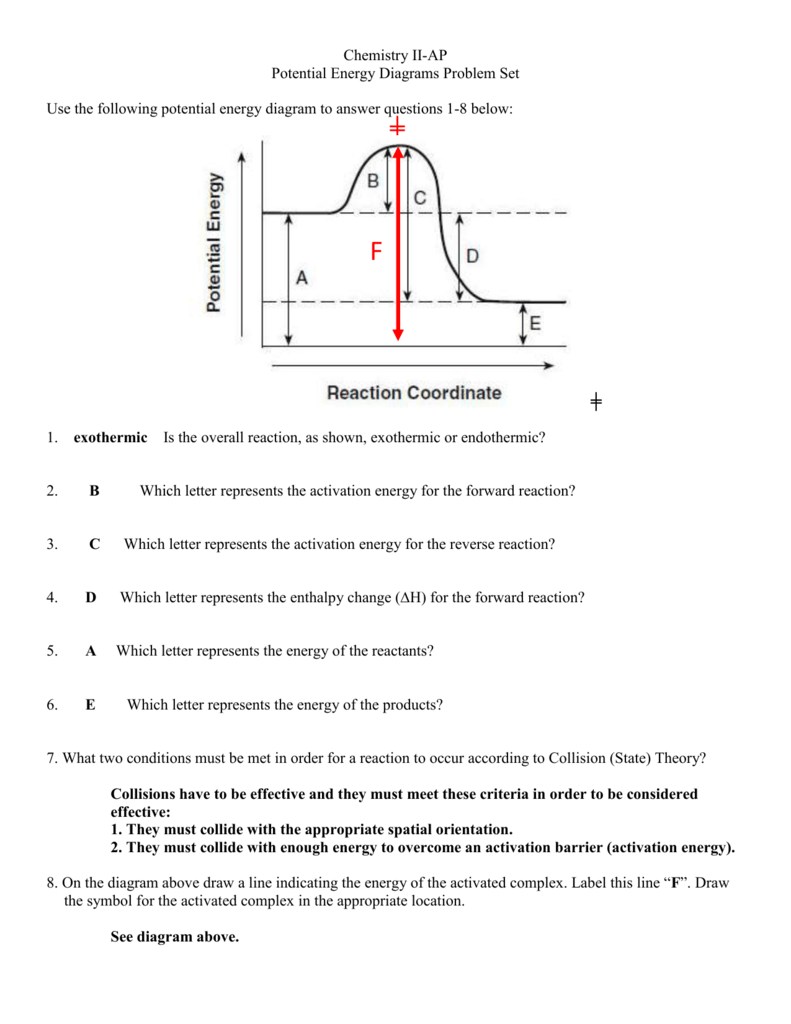
Energy Diagrams of Reactions | Fiveable Energy Diagrams. Exothermic Reactions. Endothermic Reactions. Example. 6.3 Kinetic Energy, Heat Transfer, and Thermal Equilibrium. 6.4 Heat Capacity and Coffee-Cup Calorimetry. 6.5 Phase Changes and Energy. 6.6 Introduction to Enthalpy of Reaction. 6.7 Bond Enthalpy and Bond Dissociation Energy. Potential Energy Diagram Worksheet - Worksheet Smart Potential energy diagram worksheet 1. Draw a line segment that represents the potential energy of the reactants and label the line. Draw an energy diagram for a reaction. Nat is tne cnmatpy change ah tor the d what is the enthalpv ch forward reaction. Is the overall reaction as shown exothermic or endothermic.
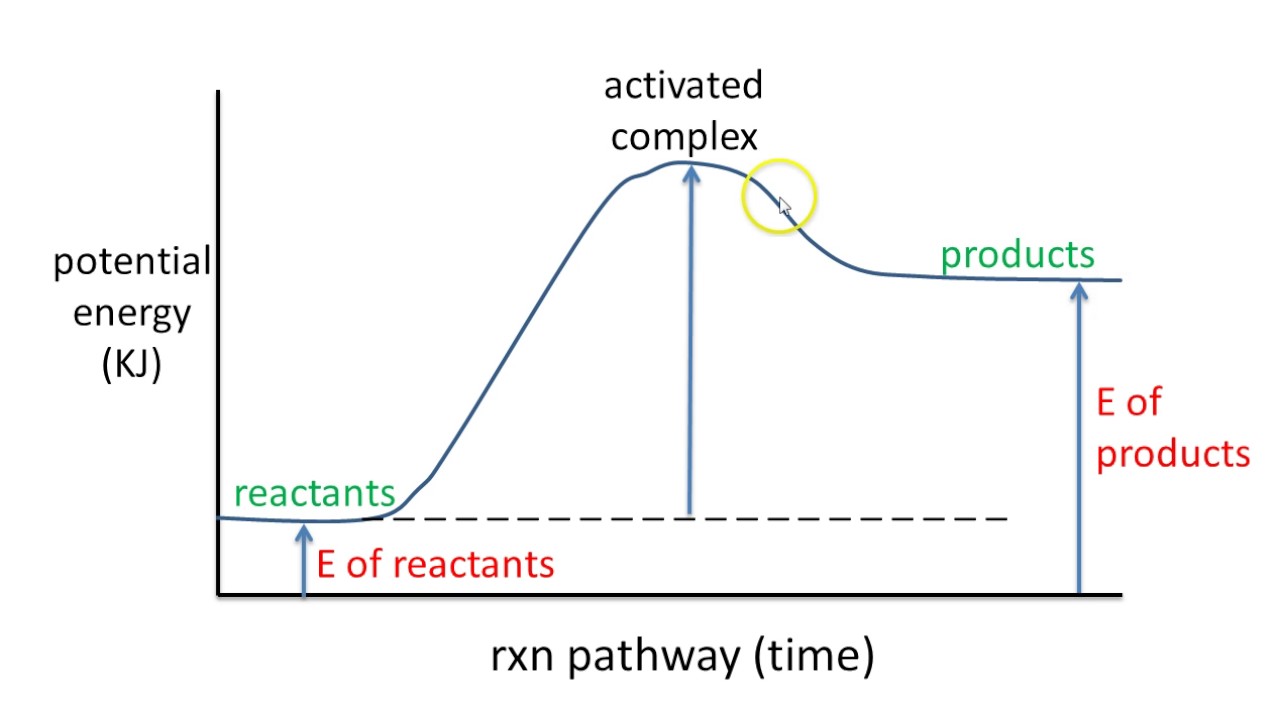
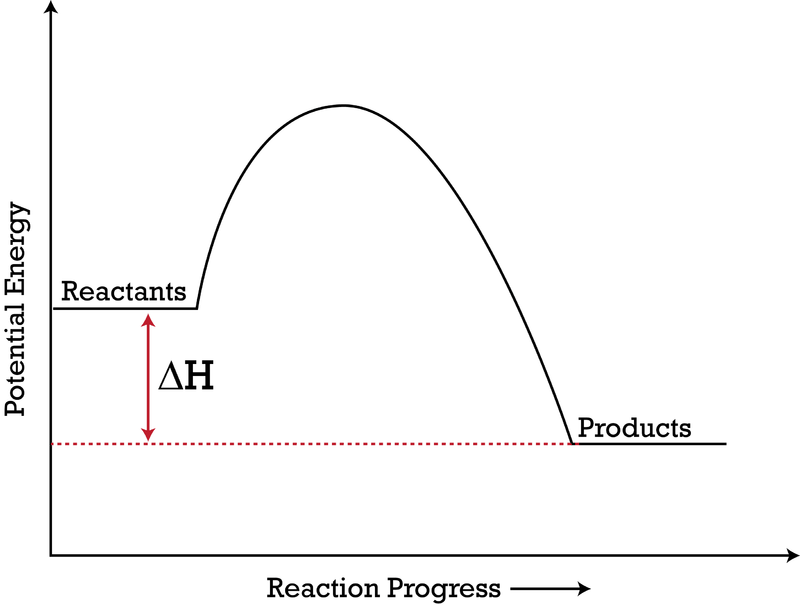
Potential Energy Diagrams | Chemistry for Non-Majors A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change is positive for an endothermic reaction and negative for an exothermic reaction.
Endothermic potential energy diagram
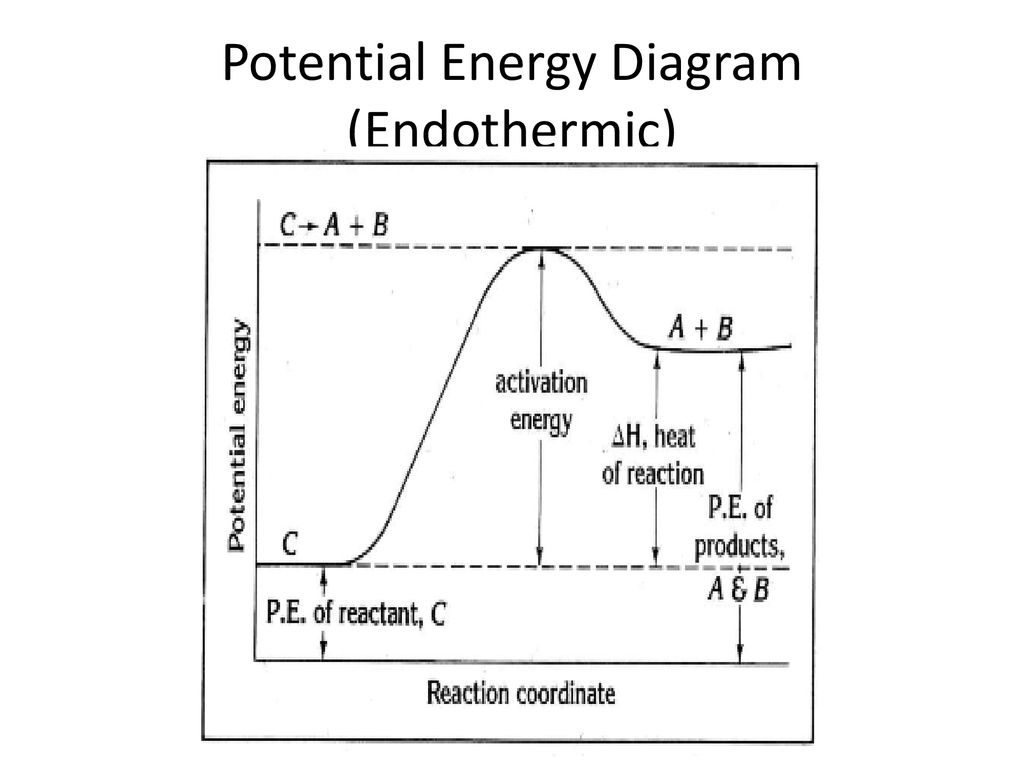
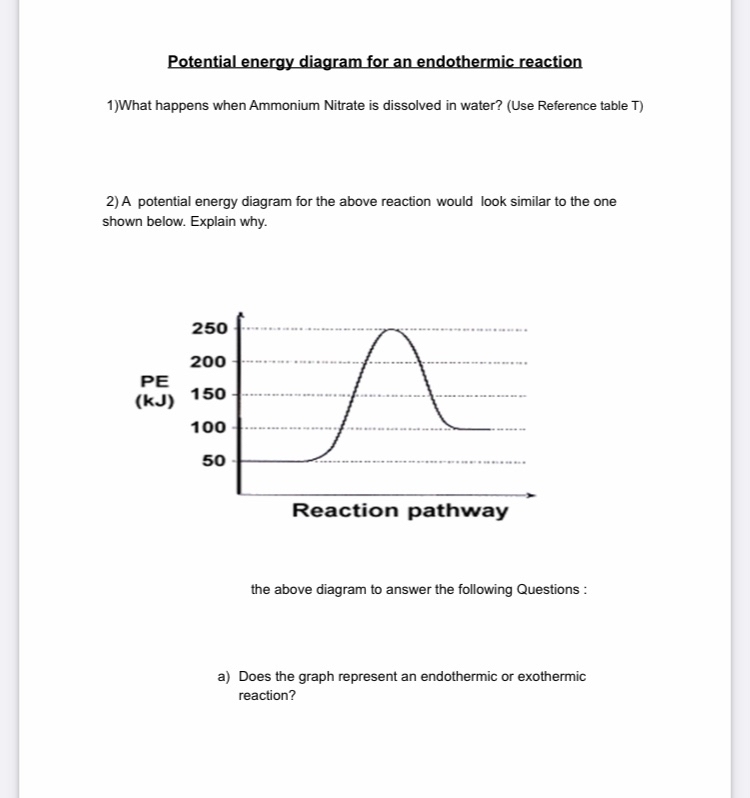
Endothermic and Exothermic Reactions Diagram | Quizlet Diagram of endothermic and exothermic reactions. Terms in this set (5) Exothermic Reaction. In this type of reaction, energy (in the form of heat, sound or light) is released when the reactants break apart. Heat energy can be picked up by the area surrounding the products. ... In endothermic reactions, there is less energy in the reactants than ... PDF Exothermic vs endothermic reaction graphs State one reason, in terms of energy, to support your answer.Answer-->Endothermic, the products have more energy than the reactants.b) On the diagram provided in your answer booklet, draw a dashed line to indicate a potential energy curve for the reaction if a catalyst is added.Answer-- Endothermic Reactions: Definition, Example, Diagram and ... Endothermic Reaction Energy Level Diagram: Endothermic reactions are depicted in a basic energy level diagram below. The activation energy is the amount of energy that must be delivered to the reactants for them to break through the energy barrier and react. In an endothermic reaction, the result has higher potential energy than the reactants.
Endothermic potential energy diagram. How To Find Highest Kinetic Energy Of Endothermic Graph ... An energy diagram illustrates how potential energy of the initial state relates to potential energy of the final state, showing how physical or chemical processes get their properties. endothermic is characterized by a higher energy rate for the starting state than the final. Representing endothermic and exothermic processes using ... A physical or chemical process can be represented using an energy diagram, which shows how the potential energy of the initial state relates to the potential energy of the final state. If the initial state has a lower potential energy than the final state, the process is endothermic. 14 Sketch a potential energy diagram for an exothermic ... 14. Sketch a potential energy diagram for an exothermic reaction and for an endothermic reaction. Label the axes, reactants, products, heat of reaction, activation energy, and transition state on each diagram. 15. The following data were obtained for the decomposition of dinitrogen pentoxide at 45 C. N 2 O 5 (mol/L) Time (min) 0.316 0 0.274 39 0.238 80 0.190 140 0.146 210 a) Plot a graph of ... Potential Energy Diagram: study guides and answers on Quizlet How does the potential-energy diagram for a reaction indicate whether the reaction is endothermic or exothermic? A. An endothermic reaction has reactants that are lower in energy than products because energy is absorbed to form the products.
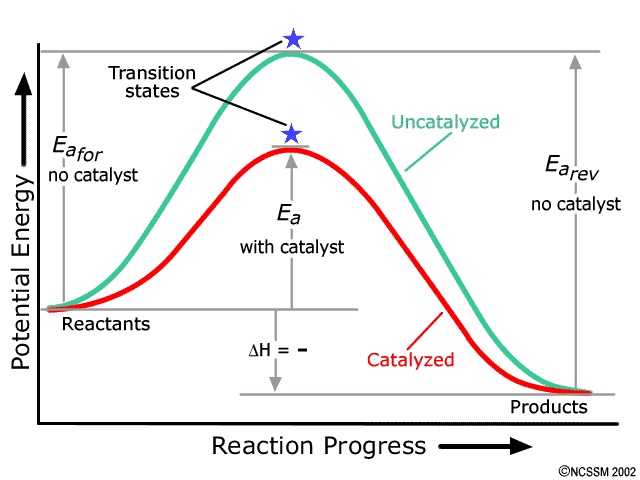
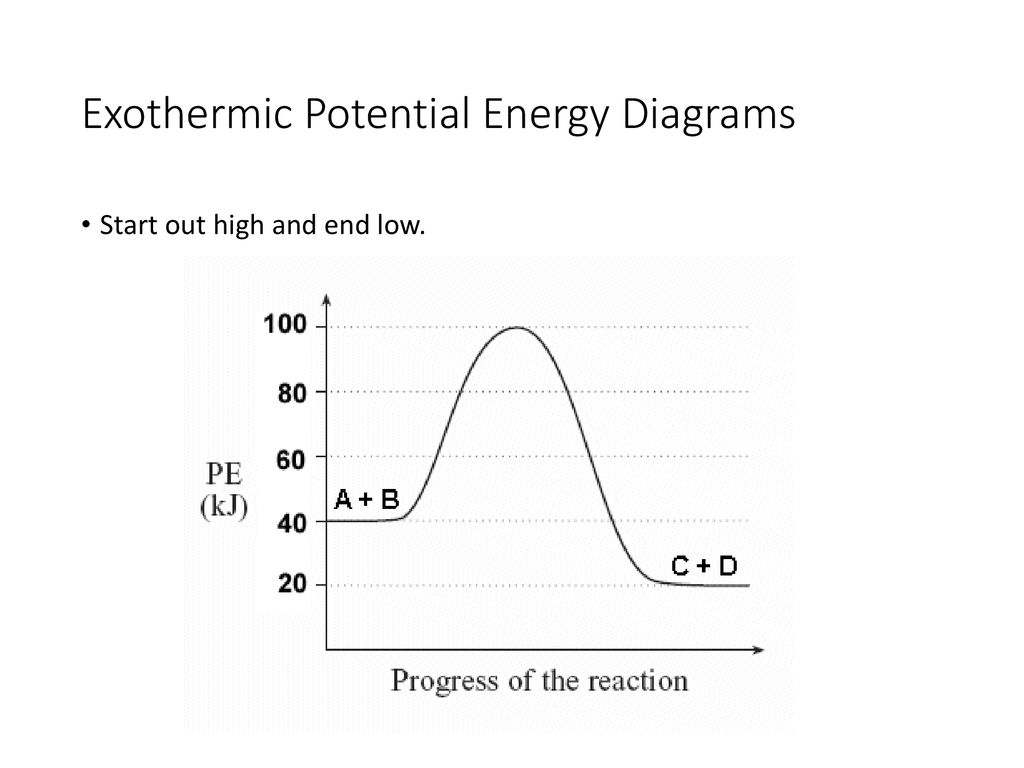
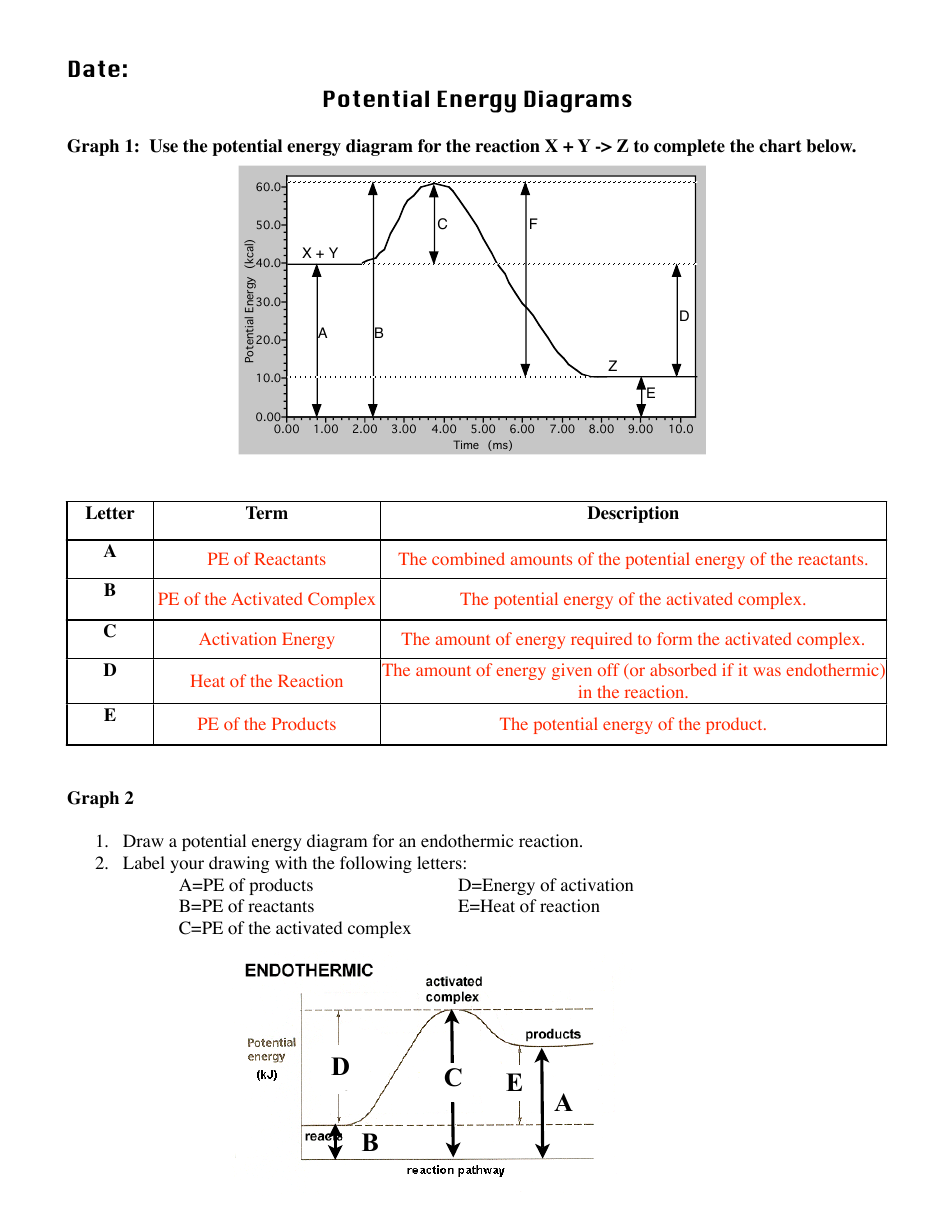
Endothermic vs Exothermic and Energy Diagrams.pdf - Name ... Label your diagram - if you are filling in electronically, click on diagram, click "edit" and fill in correct words in text boxes already provided. Key things to Note about EXOTHERMIC Energy Diagram: Reactants have MORE potential energy compared to Products Energy was released to the environment during the reaction (this is why products have less energy) Δ H will be negative Task 3 ... Endothermic and Exothermic Reactions With Potential Energy ... This chemistry video tutorial provides a basic introduction into endothermic and exothermic reactions as well as the corresponding potential energy diagrams.... PDF NAME Kinetics Potential Energy Diagrams - ISD 622 Kinetics - Potential Energy Diagrams Potential energy diagrams are used to represent potential energy changes that take place as time progresses during chemical reactions. The potential energy diagrams for the exothermic reaction between substances X and Y is shown below. The reaction progresses from stage 1 (reactants), PDF Topic 5.1 Exothermic and Endothermic Reactions Heat and ... Increase in kinetic energy - energy absorbed - endothermic Decrease in kinetic energy - energy released - exothermic Energy level diagrams Chemical Potential Energy The chemical potential energy stored in the bonds gives us a measure of a substances energy level. The higher the energy, the more chemical energy is stored in its bonds.
How to draw the potential energy diagram for this reaction ... 1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process. Endothermic vs Exothermic and Energy Diagrams.pdf - Name ... Key things to Note about ENDOTHERMIC Energy Diagram: Reactants have LESS potential energy compared to Products Energy was absorbed during the reaction (this is why the products have more energy) Δ H will be positive PDF Potential Energy Diagram Worksheet ANSWERS The reverse reaction is ____exothermic_____ (endothermic or exothermic). Reaction Rates and Potential Energy Diagrams 1. Chemical reactions occur when reactants collide. For what reasons may a collision fail to produce a chemical reaction? Not enough energy; improper angle. 2. If every collision between reactants leads to a reaction, what ... Potential Energy Diagrams - Chemistry - Catalyst ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
Potential Energy Diagrams - Kentchemistry.com 52 Sketch the potential energy diagram for an endothermic chemical reaction that shows the activation energy and the potential energy of the reactants and the potential energy of the products. Answer--> 1/04. 16 Which statement best explains the role of a catalyst in a chemical reaction?
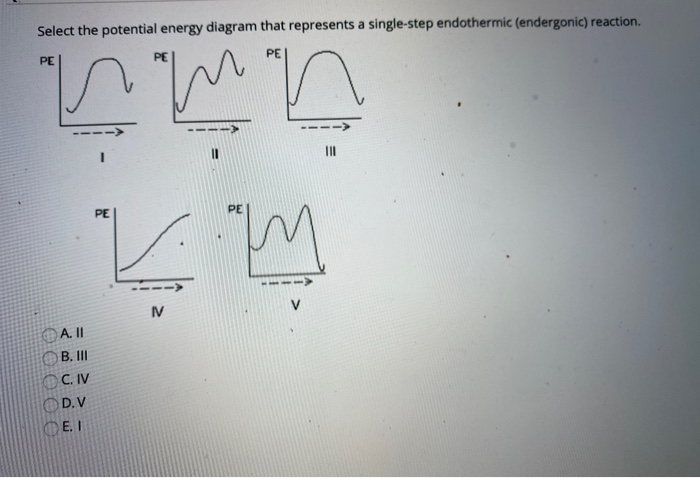
Which statement describes the potential energy diagram of ... Answer: option C. The potential energy of the products is greater than the potential energy of the reactants. Explanation: 1) The potential energy diagram shows the evolution of the potential chemical energy of a process or reaction, measured as energy of the chemical bonds, including the reactants, the intermediates, the activated complex, and the products.
What is a potential energy diagram? - Moorejustinmusic.com What is a potential energy diagram? A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. Potential energy diagrams for endothermic and exothermic reactions are described. How do you define kinetic and potential energy?
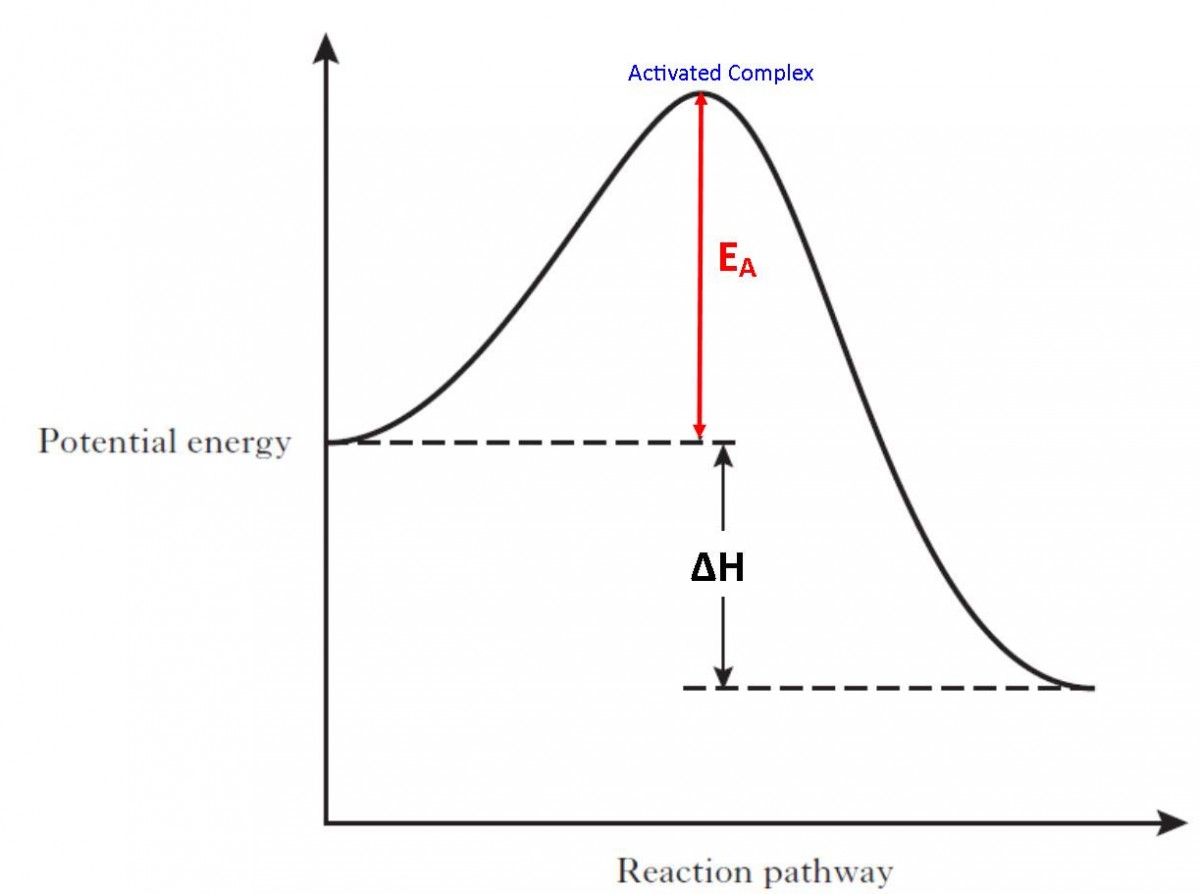
PDF Potential Energy Diagrams POTENTIAL ENERGY DIAGRAMS ARE A VISUAL REPRESENTATION OF POTENTIAL ENERGY IN A CHEMICAL REACTION ... endothermic) Activation energy is the energy required to "jump start" the reaction. Its the energy added to initiate reaction. The energy requirements can be reduced
What are Endothermic Reactions? (with Examples & Video) Energy Level Diagram of an Endothermic Reaction. The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is ...
Endothermic vs. exothermic reactions (article) | Khan Academy Energy diagrams for endothermic and exothermic reactions In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other words, the products are less stable than the reactants.
Solved If we draw a potential energy diagram for an ... The potential enegy of reactants is less… View the full answer Transcribed image text : If we draw a potential energy diagram for an endothermic reaction, the potential energy of reactant is less than potential energy of product equal to the potential energy of the product more than potential energy of product equal to activation energy of ...
How does the energy level diagram show this reaction is ... In an endothermic reaction, the reactants absorb heat energy from the surroundings to form products. Thus, the products formed have more energy than the reactans, H products > H reactants. Therefore, ΔH is positive. Energy level diagrams are used to shows the energy content of chemicals before and after a reaction. They show:
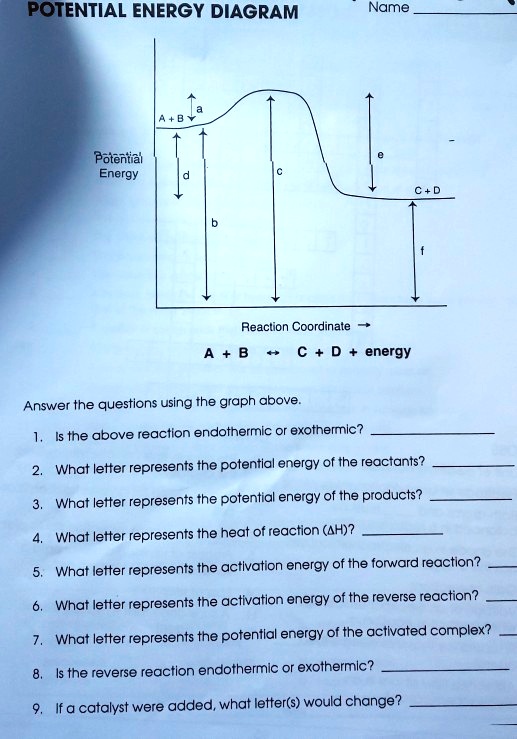
PDF Representing a Reaction with a Potential Energy Diagram Representing a Reaction with a Potential Energy Diagram (Student textbook page 371) 11. Complete the following potential energy diagram by adding the following labels: an appropriate label for the x-axis and y-axis, E a(fwd), E a(rev), ΔH r. a. Is the forward reaction endothermic or exothermic? b.
Welcome to CK-12 Foundation | CK-12 Foundation Exothermic and Endothermic Potential Energy Diagrams There are two types of potential energy diagrams. These two types center on the difference between the energies of the reactants and products. Consider the figure below. An endothermic reaction is shown on the left, and an exothermic reaction is shown on the right. ...
Potential Energy Diagrams Chemistry Catalyst Endothermic ... Potential energy diagrams chemistry catalyst, endothermic & exothermic reactions. this chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. it also shows the this chemistry video tutorial provides a basic introduction into endothermic and exothermic reactions as well as the corresponding in ...
Endothermic Reactions: Definition, Example, Diagram and ... Endothermic Reaction Energy Level Diagram: Endothermic reactions are depicted in a basic energy level diagram below. The activation energy is the amount of energy that must be delivered to the reactants for them to break through the energy barrier and react. In an endothermic reaction, the result has higher potential energy than the reactants.
PDF Exothermic vs endothermic reaction graphs State one reason, in terms of energy, to support your answer.Answer-->Endothermic, the products have more energy than the reactants.b) On the diagram provided in your answer booklet, draw a dashed line to indicate a potential energy curve for the reaction if a catalyst is added.Answer--
Endothermic and Exothermic Reactions Diagram | Quizlet Diagram of endothermic and exothermic reactions. Terms in this set (5) Exothermic Reaction. In this type of reaction, energy (in the form of heat, sound or light) is released when the reactants break apart. Heat energy can be picked up by the area surrounding the products. ... In endothermic reactions, there is less energy in the reactants than ...






















Comments
Post a Comment