42 enthalpy diagram exothermic
Enthalpy change of reactions | O Level Chemistry Notes Because enthalpy change is a measurement of energy, it can be given in the SI unit joules, J. However, it is more commonly given in kilojoules, kJ or kilojoules per mole of a substance, kJ/mol. H 2 (g) + O 2 (g) H 2 O (l), Δ H = -286 kJ/mol. Exothermic reactions have negative enthalpy change. The negative sign signifies the loss of energy when ... Enthalpy Change for Exothermic and ... - AUS-e-TUTE Enthalpy Change for Exothermic and Endothermic Reactions Chemistry Tutorial Key Concepts. The enthalpy of a chemical system refers to the "heat content" of the system. 1; Enthalpy is given the symbol H Enthalpy change refers to the amount of heat released or absorbed when a chemical reaction occurs at constant pressure.
Solved Question 22 3.33 pts Consider the enthalpy diagram ... Transcribed image text: Question 22 3.33 pts Consider the enthalpy diagram of an exothermic reaction. Where are the reactants in relation to the products on the H scale (y axis)? Oreatants are above the products reactants are below the products Question 23 3.33 pts For an exothermic reaction heat is considered a enthalpy is a number. and the change in reactant; negative O product; negative ...

Enthalpy diagram exothermic
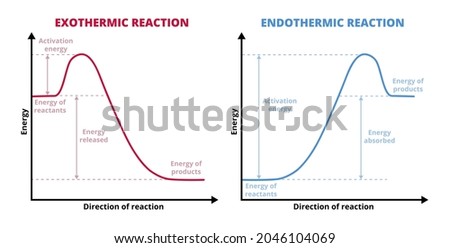
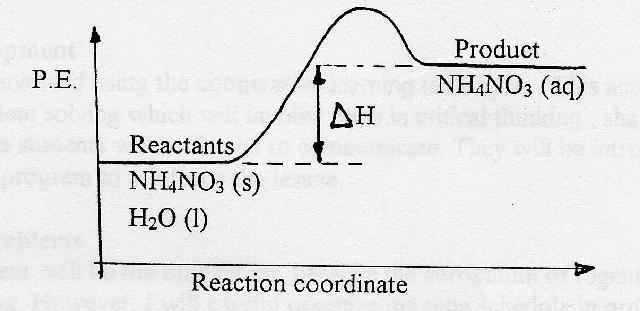
chem.libretexts.org › Enthalpy_of_SolutionEnthalpy of Solution - Chemistry LibreTexts Feb 16, 2021 · The enthalpy of solution can expressed as the sum of enthalpy changes for each step: \[ΔH_{solution} = ΔH_1 + ΔH_2 + ΔH_3. \label{eq1}\] So the enthalpy of solution can either be endothermic, exothermic or neither \(ΔH_{solution} = 0\)), depending on how much heat is required or release in each step. Exothermic Energy Diagram: Activation Energy, Transition ... In this video, I go over how to properly label and explain a reaction mechanism diagram which is also referred to as an energy diagram or energy graph. I'll ... byjus.com › chemistry › endothermic-reactionWhat are Endothermic Reactions? (with Examples & Video) The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is generally lower than that of the reactant.
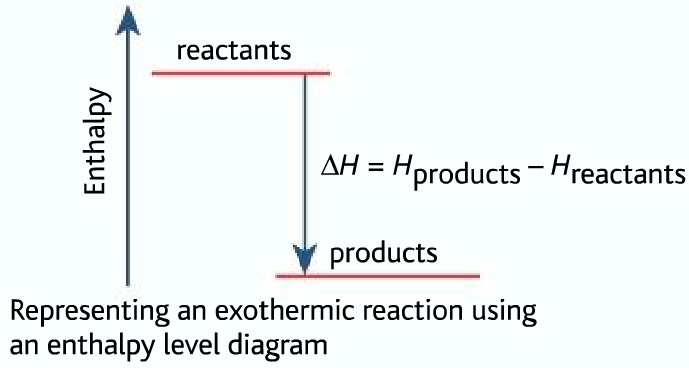
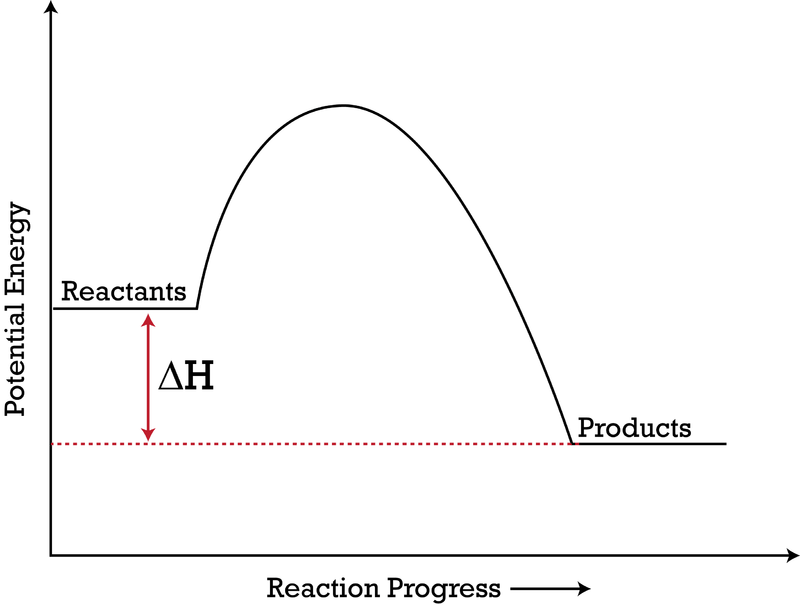
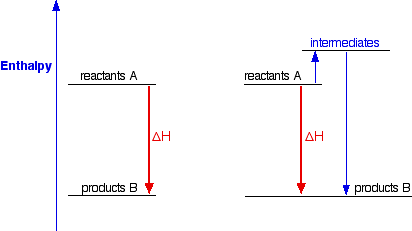
Enthalpy diagram exothermic. What is the enthalpy of reaction from the following energy ... From the given energy diagram we conclude that this is an exothermic reaction diagram. Thus, the enthalpy of reaction is, -20 kJ. The enthalpy of reaction is the amount of heat that is required or to be released in order to convert the reactants to the products. this can be calculated through the equation, δh = h (products) - h (reactants) Chemistry 124 Chapter 6: Thermodynamics (Jursich) - Quizlet -Enthalpy, H, increases up the y-axis. -In an endothermic reaction, the reactants will be at the bottom of the enthalpy diagram. -The arrow in the enthalpy diagram points from reactants to products. -The direction of the arrow in an enthalpy diagram indicates whether a reaction is exothermic or endothermic. How to Draw & Label Enthalpy Diagrams - Video & Lesson ... An enthalpy diagram is a method used to keep track of the way energy moves during a reaction over a period of time. Learn how to draw and label enthalpy diagrams, the definition of an enthalpy ... Energy diagrams - Energy changes in chemical reactions ... An energy level diagram for an exothermic reaction In an endothermic reaction, the products are at a higher energy than the reactants. This means that the enthalpy change of the reaction (∆ H ...
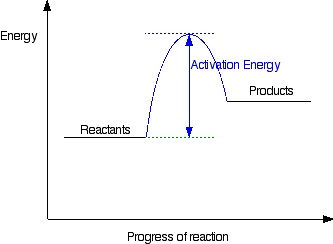
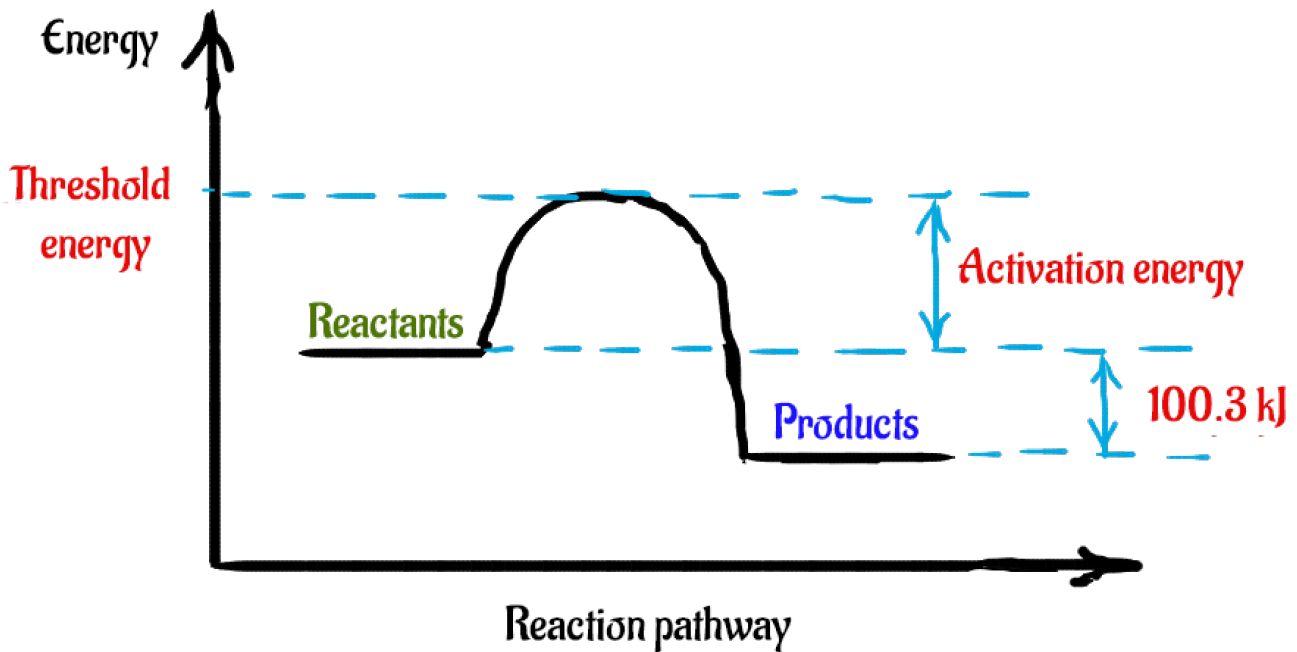
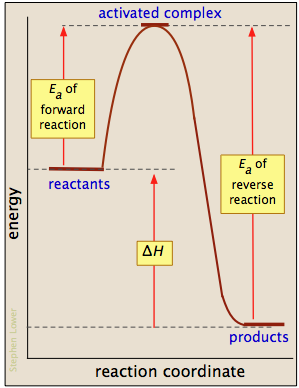
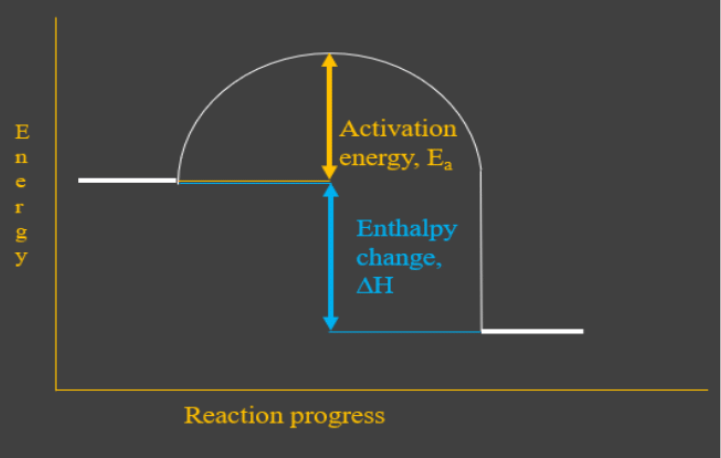
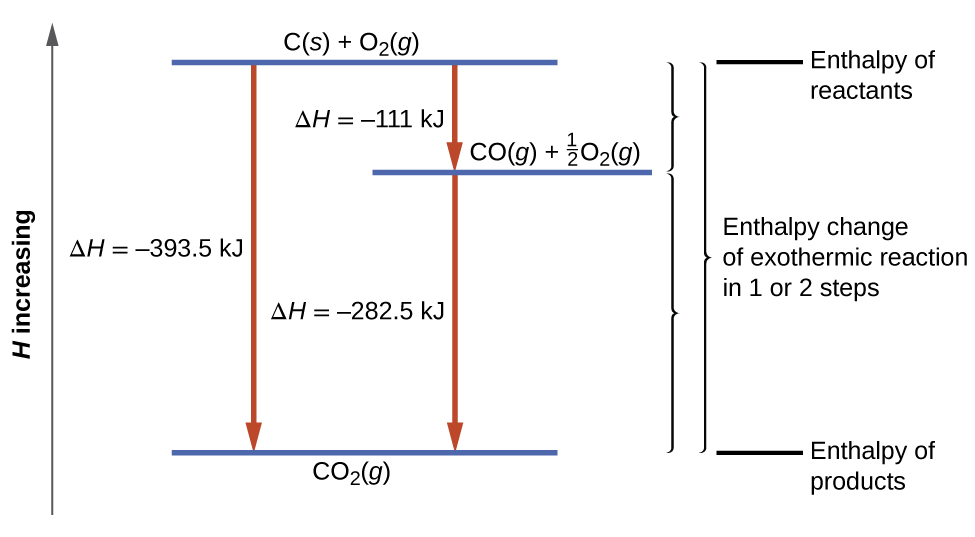
How do you know if a diagram is endothermic or exothermic ... In the energy level diagram, the enthalpies of the products are lower than that of the reactants. Hence, the enthalpy change is negative (ΔH<0). By examining this enthalpy change, one can tell whether a reaction is endothermic (ΔH>0) or exothermic (ΔH<0). Energy From Chemicals - Enthalpy Change - Difference ... Energy Profile diagrams: Energy profile diagrams are used to represent the energy pathway from reactants to products in a reaction. The energy profile diagram for exothermic reaction show that the reactants have higher initial energy and products have lower energy since the energy has been given out during the reaction. Energy Diagrams of Reactions | Fiveable Energy Diagrams. Exothermic Reactions. Endothermic Reactions. Example. 6.3 Kinetic Energy, Heat Transfer, and Thermal Equilibrium. 6.4 Heat Capacity and Coffee-Cup Calorimetry. 6.5 Phase Changes and Energy. 6.6 Introduction to Enthalpy of Reaction. 6.7 Bond Enthalpy and Bond Dissociation Energy. PDF Topic 5.1 Exothermic and Endothermic Reactions Heat and ... c) Draw a labeled enthalpy level diagram for an exothermic and endothermic reaction showing the activation energy, Ea and enthalpy change. [4] 9. (M05/S/2) In a neutralization reaction 50 cm 3 of a 0.50 moldm-3 solution of sodium hydroxide is mixed rapidly in a glass beaker with 50 cm 3 of a 0.050 moldm-3 solution of sulfuric acid.
SOLVED:Interpret Scientific Illustrations The reaction A ... Describe exothermic and endothermic reactions and factors that affect the ra… 01:13 Draw an enthalpy diagram for a general endothermic reaction; label the axis,… PDF Exothermic enthalpy diagram - Tangente Mag Exothermic enthalpy diagram In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation. Enthalpy change is one of the most important concepts in chemistry. It does not matter what level of chemistry you are learning, your concepts of enthalpy change must be clear. Draw the enthalpy diagram for exothermic and endothermic ... The enthalpy diagram for exothermic and endothermic reactions is shown below. Explanation : Endothermic reaction : It is defined as the chemical reaction in which the energy is absorbed from the surrounding. In the endothermic reaction, the energy of reactant are less than the energy of product. Amazing Enthalpy Diagram For Exothermic Reaction Important ... Define the terms exothermic reaction endothermic reaction and standard enthalpy change of reaction. Draw an enthalpy diagram for a general exothermic reaction. For Exothermic reaction ΔH is negative it means the system loses energy to the surroundings. It also shows the effect of a catalyst on the f. Therefore ΔH is negative ΔH.
PDF 5.1 - Exothermic and Endothermic Reactions 5.1.4 - Deduce, from an enthalpy level diagram, the relative stabilities of reactants and products, and the sign of the enthalpy change for the reaction Enthalpy level diagrams, or energy profile diagrams, allow us to visualise what happens to the enthalpy of a reaction as it proceeds The total enthalpy of the reactant species is labelled H R
Creative Diagram Of Exothermic Reaction - Glaucoma Template Gibbs free energy and spontaneity. So the activation energy is the minimum amount of energy required for a reaction to take place. Heat Of Reaction Reflects The Difference In Enthalpy Between The Products And The Reactants Teaching Chemistry Chemistry Education Teaching Science The energy profile diagram of an exothermic reaction is given below.
PDF Enthalpy Changes - WordPress.com Energy level diagram - exothermic These show the relative energy levels (enthalpy) of reactants and products: H 2 + Cl 2 (products) Enthalpy exothermic 2HCl (reactants) Note that the specific reactants and products are shown for the reaction taking place.
CHM 115 LearnSmart Chapter 6 Flashcards - Quizlet - Enthalpy, H, increase up the y-axis - The arrow in the enthalpy diagram points from reactants to products - The direction of the arrow in an enthalpy diagram indicates whether a reaction is exothermic or endothermic - In an endothermic reaction, the reactants will be at the bottom of the enthalpy diagram
Endothermic vs. exothermic reactions (article) | Khan Academy Phase diagrams. Enthalpy. Heat of formation. Hess's law and reaction enthalpy change. Gibbs free energy and spontaneity. Gibbs free energy example. More rigorous Gibbs free energy / spontaneity relationship. A look at a seductive but wrong Gibbs spontaneity proof. Endothermic vs. exothermic reactions.
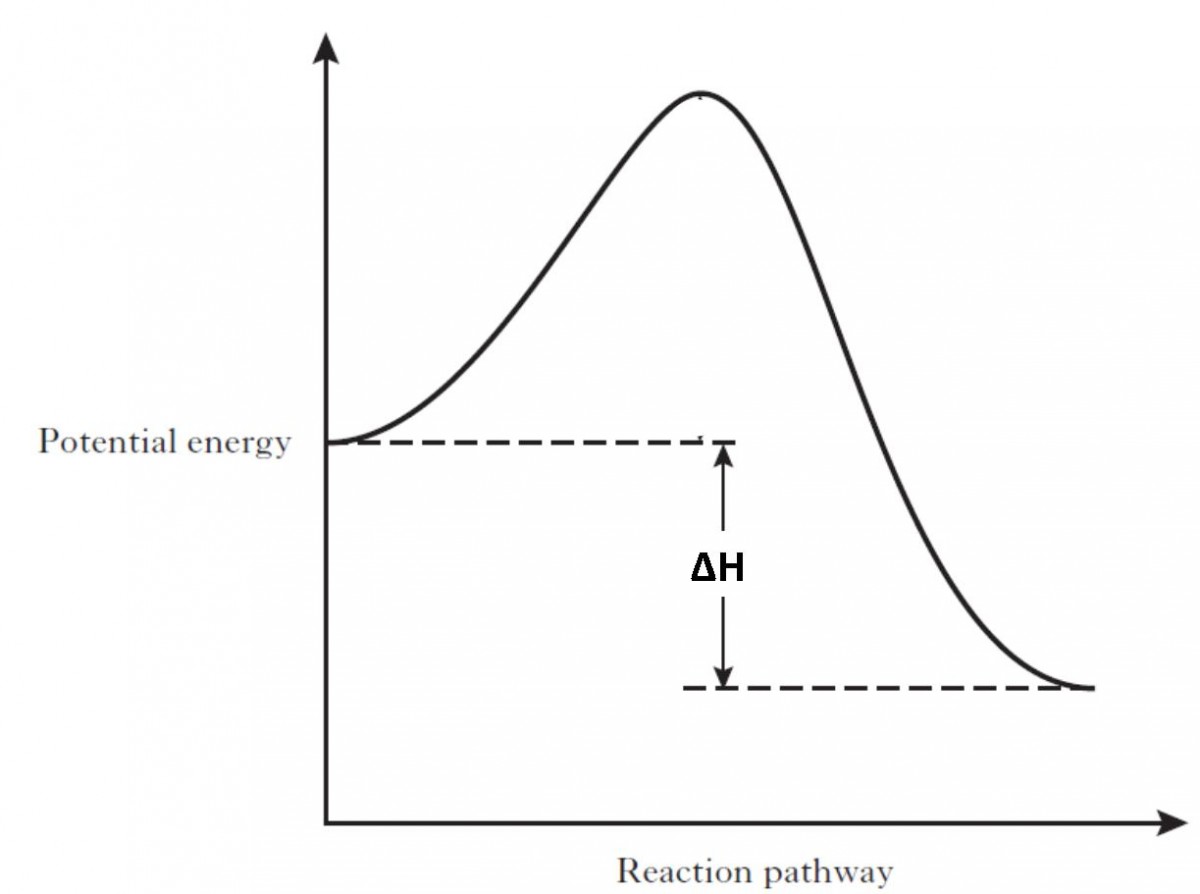
Reaction profiles - Exothermic and endothermic reactions ... The diagram shows a reaction profile for an exothermic reaction. A reaction profile for an exothermic reaction Question. Describe how you can tell from a reaction profile that a reaction is ...
What is the enthalpy change for reverse reaction ... An enthalpy diagram plots information about a chemical reaction such as the starting energy level, how much energy needs to be added to activate the reaction, and the ending energy. An enthalpy diagram is graphed with the enthalpy on the y-axis and the time, or reaction progress, on the x-axis.
File:Enthalpy-Diagram-Solution-Formation-Exothermic.svg ... File:Enthalpy-Diagram-Solution-Formation-Exothermic.svg. Size of this PNG preview of this SVG file: 409 × 195 pixels. Other resolutions: 320 × 153 pixels | 640 × 305 pixels | 1,024 × 488 pixels | 1,280 × 610 pixels | 2,560 × 1,221 pixels.
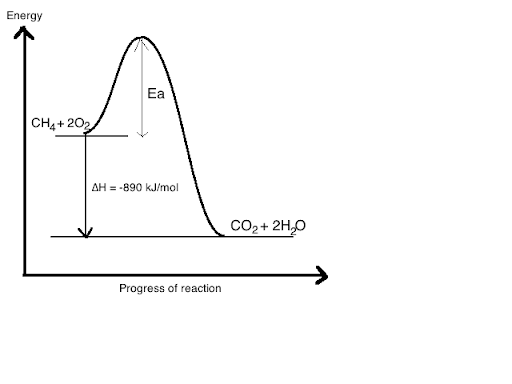
Solved Write a balanced equation and draw an enthalpy ... This problem has been solved! Write a balanced equation and draw an enthalpy diagram for (select if exothermic or endothermic): [ Select ] ["endothermic", "exothermic"] combustion of one mole of methane. [ Select ] ["endothermic", "exothermic"] vaporization of liquid alcohol.
Enthalpy Diagram For Endothermic Reaction - Diagram ... Enthalpy diagram for endothermic reaction. To use the data for exothermic bond forming we have to make it negative. A look at a seductive but wrong gibbs spontaneity proof. Enthalpy change for endothermic reactions. This can be seen in the potential energy diagrams.
Enthalpy Diagrams | Pathways to Chemistry In this video we draw enthalpy diagrams for an exothermic reaction. In this video we draw enthalpy diagrams for an exothermic reaction. Pathways to Chemistry Chemistry for College, Middle and High School, Homeschooled Students, Teachers and Parents. ... 1 thought on " Enthalpy Diagrams "
Bond Enthalpy: Definition, Reactions, Diagrams Exothermic Process - Reactions in which the reactant products are having much higher energy than the reaction products and the change in enthalpy remains always negative (H <0). The following diagram will help us to understand the two processes: Endothermic Reaction. Exothermic Reaction. Bond enthalpy: Hydrogenation of a Propene
byjus.com › chemistry › endothermic-reactionWhat are Endothermic Reactions? (with Examples & Video) The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is generally lower than that of the reactant.
Exothermic Energy Diagram: Activation Energy, Transition ... In this video, I go over how to properly label and explain a reaction mechanism diagram which is also referred to as an energy diagram or energy graph. I'll ...
chem.libretexts.org › Enthalpy_of_SolutionEnthalpy of Solution - Chemistry LibreTexts Feb 16, 2021 · The enthalpy of solution can expressed as the sum of enthalpy changes for each step: \[ΔH_{solution} = ΔH_1 + ΔH_2 + ΔH_3. \label{eq1}\] So the enthalpy of solution can either be endothermic, exothermic or neither \(ΔH_{solution} = 0\)), depending on how much heat is required or release in each step.













![5.3 Deduce, via enthalpy level diagrams the stabilitiy of reactants and products [SL IB Chemistry]](https://i.ytimg.com/vi/ijdhORfkOY0/maxresdefault.jpg)







Comments
Post a Comment