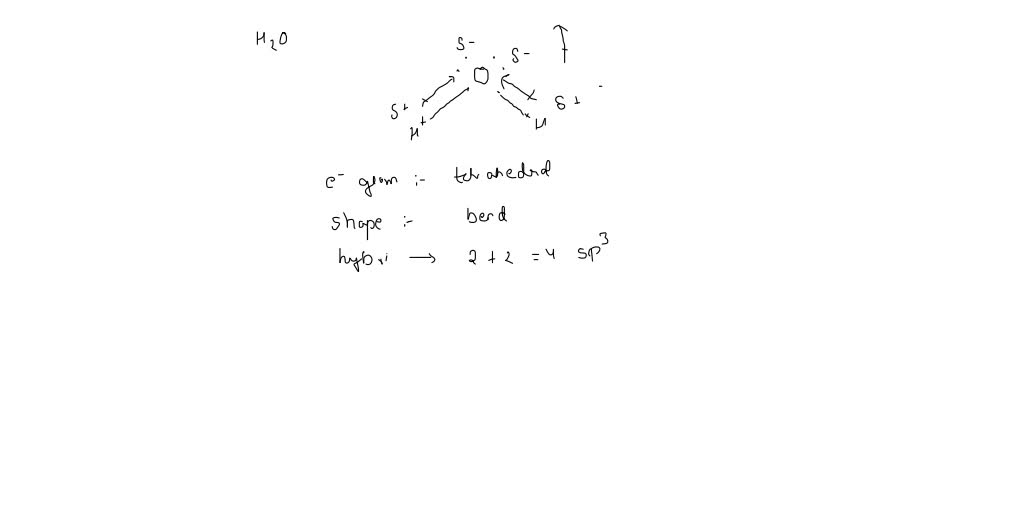
40 make an electron distribution diagram of water
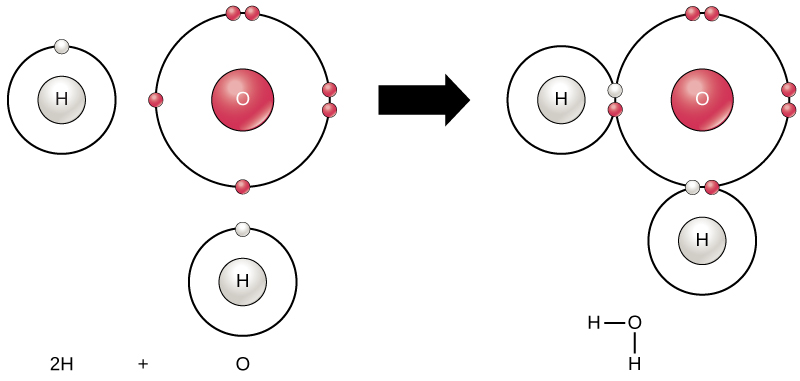
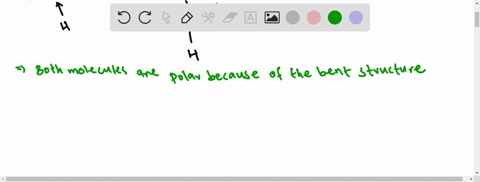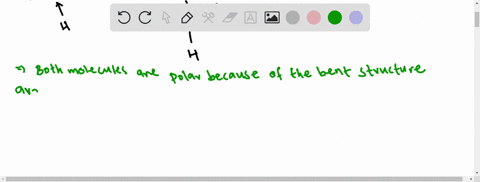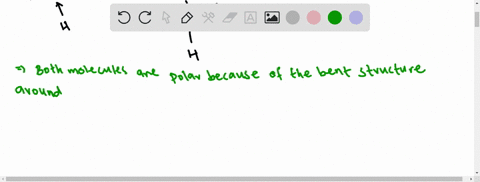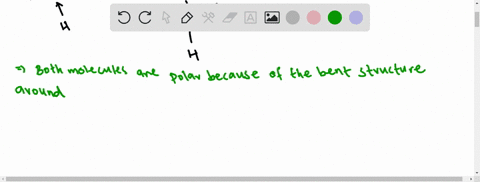
3.1.4 Draw and label a diagram showing the structure of ... Water (H2O) should be drawn as two hydrogen atoms connected to one oxygen atom by a bond known as a polar covalent bond. There are two lone pairs of electron... 40 electron distribution diagram of water - Diagram For You Jan 14, 2022 · 40 electron distribution diagram of water. Written By Rosa B. Pruitt Friday, January 14, 2022 Add Comment. Edit. 4 answersyou know That H 20. There's one water molecule which is six electrons and it's valid shell. It first make a bond with hydrogen And there are two lone pairs. This photo about: Lewis Dot Diagram for Water, entitled as Electron ...
Electron Configuration Diagrams | Properties of Matter ... Electron Configuration Diagrams | Properties of Matter | Chemistry | FuseSchoolLearn the basics about Drawing electron configuration diagrams. Find out more ...

Make an electron distribution diagram of water
Electron distribution in water | Request PDF The distribution of electron density in a water molecule is very nearly spherical, and orientational correlation between molecules in the liquid is not "seen" by x rays. Structure and correlation ... PDF Welcome to AP Biology. I look forward to working with you ... 18. Make an electron distribution diagram of water. Which element is most electronegative? Why is water considered a polar molecule? Label the regions that are more positive or more negative. (This is a very important concept. Spend some time with this one!) PDF Chapter 2: The Chemical Context of Life 18. Make an electron distribution diagram of water. Which element is most electronegative? Why is water considered a polar molecule? Label the regions that are more positive or more negative. (This is a very important concept. Spend some time with this one!)
Make an electron distribution diagram of water. Water | H2O - PubChem Water is an oxygen hydride consisting of an oxygen atom that is covalently bonded to two hydrogen atoms. It has a role as an amphiprotic solvent, a member of greenhouse gas, a human metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite. Electron distribution in water: The Journal of Chemical ... May 17, 2000 · The x-ray structure factor of water measured under ambient conditions with synchrotron radiation is compared with those predicted on the basis of partial structure factors describing the nuclear positions obtained by neutron diffraction and of different assumptions for the electron distribution. PDF Sodium Chlorine Sodium chloride Electron orbitals 7 Neon, with two filled Shells (10 electrons) First shell Second shell First shell Second shell 1s orbital 2s orbital Three 2p orbitals (a) Electron distribution diagram (b) Separate electron orbitals (c) Superimposed electron orbitals 1s, 2s, and 2p orbitals x y z 8 Electron Distribution Diagram | Electron Distribution ... Friend. Family. Unfollow. Electron Distribution Diagram. Electron Distribution Table for my Bio 111 class. Done. Show your appreciation with the gift of Flickr Pro. Comment. 5,107 views.
PDF Molecular Physics The structure of ambient water charge distribution and hence the atomic form fac-tors [6]. For example, ab initio simulation studies of water report that the electron distribution around a single water molecule is much changed from the gas phase, with more charge residing on the oxygen and with a more spherical distribution of charge [46]. Once PDF Water: Structure and Introductory article Properties Water: Structure and Properties ... electron orbitals overlap. At larger distances two atoms ... than that of hydrogen the electron distribution is concentrated more around the former, i.e. water is electricallypolarized,havingapermanentdipolemoment of 6 10230Cm in the gas phase. The dipole moment is Chapter 2: The Chemical Context of Life Flashcards - Quizlet For example, the bond between the oxygen and hydrogen atoms of a water molecule is a polar covalent bond. Make an electron distribution diagram of water. Which element is most electronegative? Why is water considered a polar molecule? Label the regions that are more positive or more negative. (This is a very important concept. PDF Chapter 2: The Chemical Context of Life - Copley 18. Make an electron distribution diagram of water. Which element is most electronegative? Why is water considered a polar molecule? Label the regions that are more positive or more negative. (This is a very important concept. Spend some time with this one!) 19. Another bond type is the ionic bond. Explain what is happening in the figure below ...
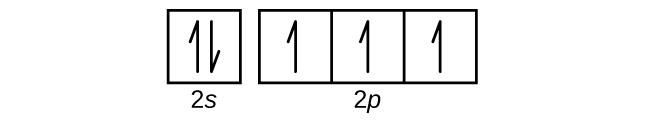
Carbon(C) electron configuration and orbital diagram Carbon (C) excited state electron configuration and orbital diagram. Then correct electron configuration of carbon in ground state will be 1s 2 2s 2 2p x1 2p y1. Therefore, the valency of carbon is 2. Also, the valency of an element is determined by electron configuration in the excited state. make an electron distribution diagram of water. Which elemen make an electron distribution diagram of water. Subject: Biology Price: Bought 3. Share With. make an electron distribution diagram of water. Which element is most electronegative? Why is water considered a polar molecule? Label the regions that are more positive or more negative macromolecules Fall 20 (1).pdf - LAB EXERCISE: Chemistry 1 ... The figure below represents an electron distribution diagram where the concentric circles represent electron shells and each small circle represents an empty spot for an electron. Fill out the electron distribution diagram for the element carbon by coloring the electrons that are present; then answer the questions. Answered: Draw the electron distribution diagram… | bartleby Answered: Draw the electron distribution diagram… | bartleby. Draw the electron distribution diagram for water. Begin with 1 central water molecule. Show the chemistry of each element within the central water molecule (all electron orbits, lone pair electrons, type of chemical bond, polarity/charge, and correct shape).
Covalent Bonding of Water (H2O) | The Ultimate Guide An ionic bond is a type of chemical bond in which the atoms have different electronegativity values from each other. For example, sodium (Na) and chlorine (Cl) form an ionic bond to make NaCl (table salt). However, in a covalent bond, the atoms are bound to share electrons. For example, if we talk about water ( H2O), it is a polar covalent bond.
PDF 1)Draw an electron-dot diagram for each of the following ... 1)Draw an electron-dot diagram for each of the following substances: a CaO(an ionic compound) b HBr c N2 Base your answers to questions 2 and 3 on the information below and on your knowledge of chemistry. The formulas and the boiling points at standard pressure for ethane, methane, methanol, and water are shown in the table below.
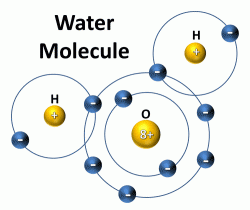
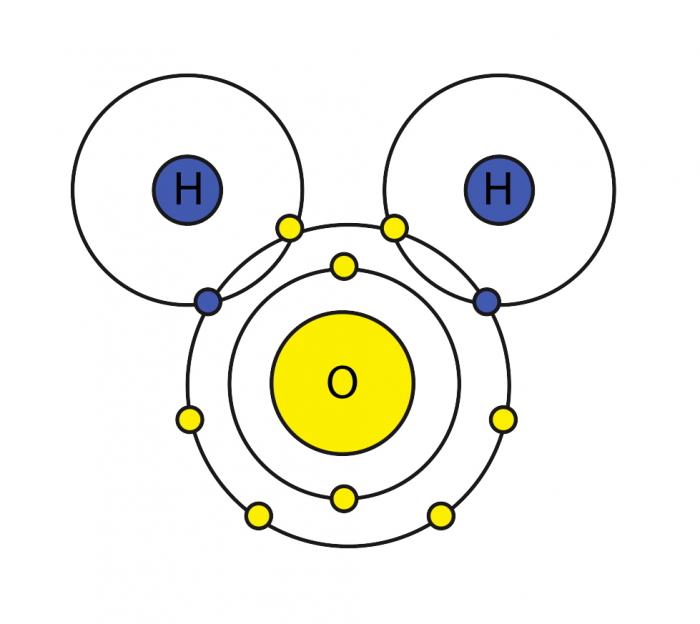
The Configuration of the Water Molecule | EARTH 111: Water ... The Configuration of the Water Molecule. A molecule of water is composed of two atoms of hydrogen and one atom of oxygen. The one and only electron ring around the nucleus of each hydrogen atom has only one electron. The negative charge of the electron is balanced by the positive charge of one proton in the hydrogen nucleus.
PDF Chapter 2: The Chemical Context of Life - WordPress.com For example, the bond between the oxygen and hydrogen atoms of a water molecule is a polar covalent bond. 20. Make an electron distribution diagram of water. Which element is most electronegative? Why is water considered a polar molecule? Label the regions that are more positive or more negative. (This is a very important concept.
PDF Chapter 4: Carbon and the Molecular Diversity of Life 3. Make an electron distribution diagram of carbon. ! Carbon has 4 valence electrons, can bond to 4 items, and typically forms covalent bonds with other elements. 4. Carbon chains form skeletons. List here the types of skeletons that can be formed. Carbon skeletons vary in length. The skeleton may have double bonds, which can vary in location.
Water and its structure - Chem1 The molecule of water. A molecule is an aggregation of atomic nuclei and electrons that is sufficiently stable to possess observable properties — and there are few molecules that are more stable and difficult to decompose than H 2 O. In water, each hydrogen nucleus is bound to the central oxygen atom by a pair of electrons that are shared between them; chemists call this shared electron pair ...
Electron distribution in water (Journal Article) | OSTI.GOV Jun 01, 2000 · The U.S. Department of Energy's Office of Scientific and Technical Information
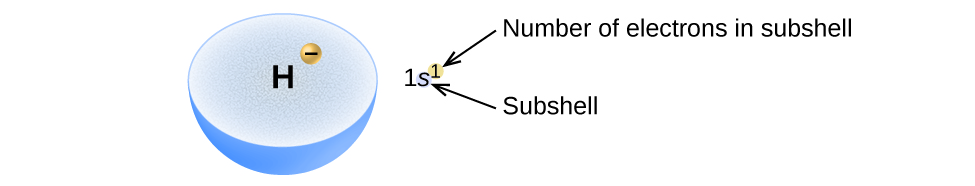
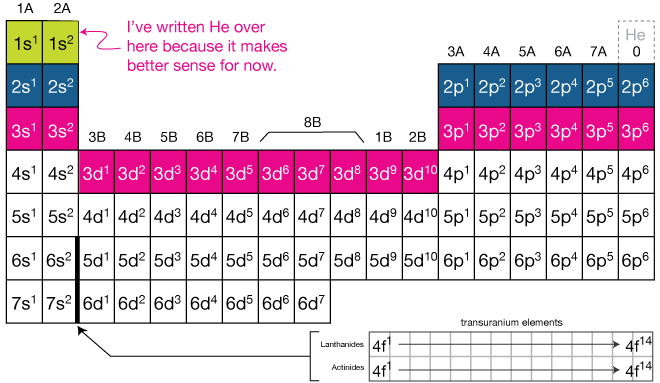
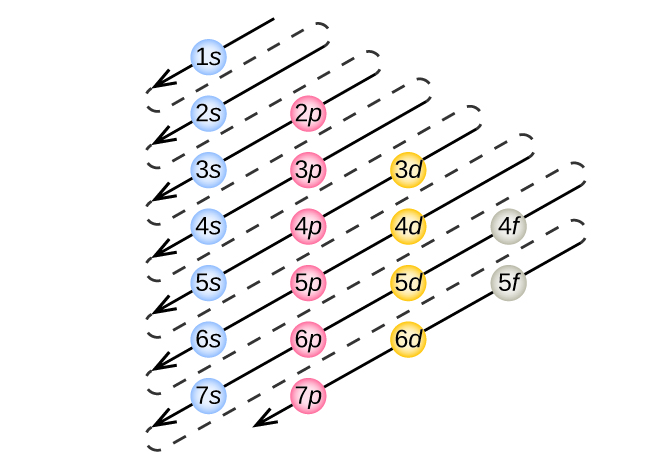
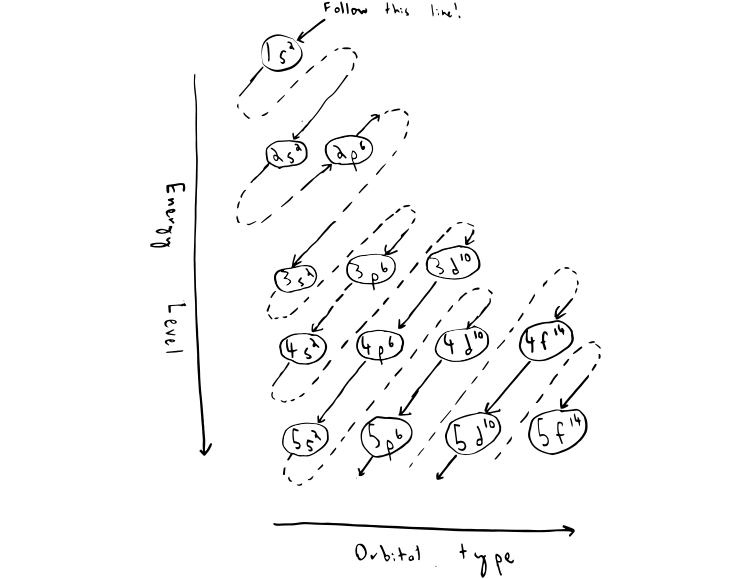
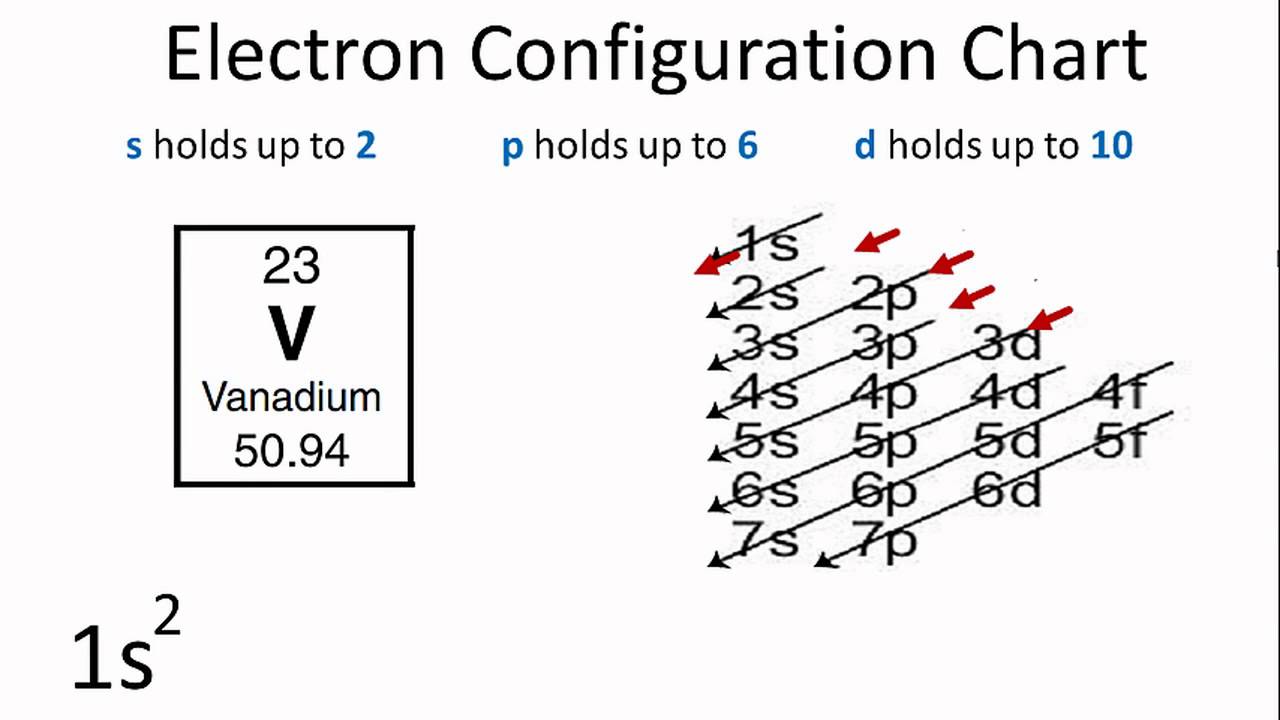
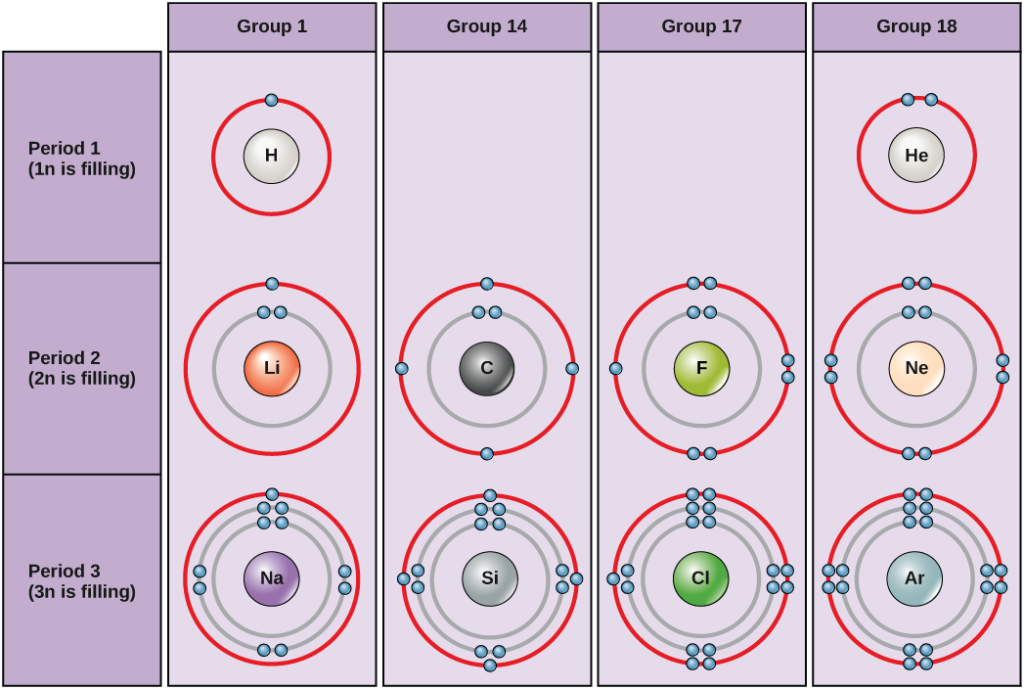
Atom Diagrams: Electron Configurations of the Elements For each electron shell atom diagram, the element symbol is listed in the nucleus. The electron shells are shown, moving outward from the nucleus. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The element atomic number and name are listed in the upper left.
Water Structure & Properties | Molecule & Physical ... Structure of Water molecule In a water molecule, each hydrogen atom shares an electron pair with the oxygen atom. The geometry of the water molecule is dictated by the shapes of the outer electron orbitals of the oxygen atom, which are similar to the bonding orbitals of carbon. These orbitals describe a rough tetrahedron, with ... -more-container">
Structure of Hydrogen, Carbon, Oxygen and Nitrogen P2: Describe the structure of water and carbon dioxide with reference to different types of bonding Water (Worldofmolecules.com, 2018) Water is a polar molecule and a chemical compound. A water molecule consists of two hydrogen atoms and an oxygen atom, its' molecular formula is H2O.
PDF !Chapter 2: The Chemical Context of Life Make an electron distribution diagram of water. Which element is most electronegative? Why is water considered a polar molecule? Label the regions that are more positive or more negative. (This is a very important concept. Spend some time with this one!) - 3 - •• 0 : 19. Another bond type is the
Types of Covalent Bonds: Polar and Nonpolar | manoa.hawaii ... Fig. 3-2: Different ways of representing the polar sharing of electrons in a water molecule. Each diagram shows the unsymmetrical shape of the water molecule. In (a) & (b), the polar covalent bonds are shown as lines. In part (c), the polar covalent bonds are shown as electron dots shared by the oxygen and hydrogen atoms.
Electrical Diagram | Free Electrical Diagram Templates This electrical diagram template can be customized and used to represent electrical circuits. Open the template with Edraw, and you can see all the circuit symbols, resistors, switches, connectors, and more. Basic electrical. 27224. 134.
Chapter_2 - Name Biology Chapter 2 Active Reading Guide ... Make an electron distribution diagram of water. Which element is most electronegative? Why is water considered a polar molecule? Label the regions that are more positive or more negative. (This is a very important concept. Spend some time with this one!) 1. Another bond type is the ionic bond. Explain what is happening in Figure 2.10.
AP Bio Chapter 2 Reading Guide Flashcards - Quizlet Make an electron distribution diagram of water. Which element is most electronegative? Why is water considered a polar molecule? Label the regions that are more positive or more negative. (This is a very important concept. Spend some time with this one!)
PDF Chapter 2: The Chemical Context of Life 18. Make an electron distribution diagram of water. Which element is most electronegative? Why is water considered a polar molecule? Label the regions that are more positive or more negative. (This is a very important concept. Spend some time with this one!)
PDF Welcome to AP Biology. I look forward to working with you ... 18. Make an electron distribution diagram of water. Which element is most electronegative? Why is water considered a polar molecule? Label the regions that are more positive or more negative. (This is a very important concept. Spend some time with this one!)
Electron distribution in water | Request PDF The distribution of electron density in a water molecule is very nearly spherical, and orientational correlation between molecules in the liquid is not "seen" by x rays. Structure and correlation ...






















Comments
Post a Comment